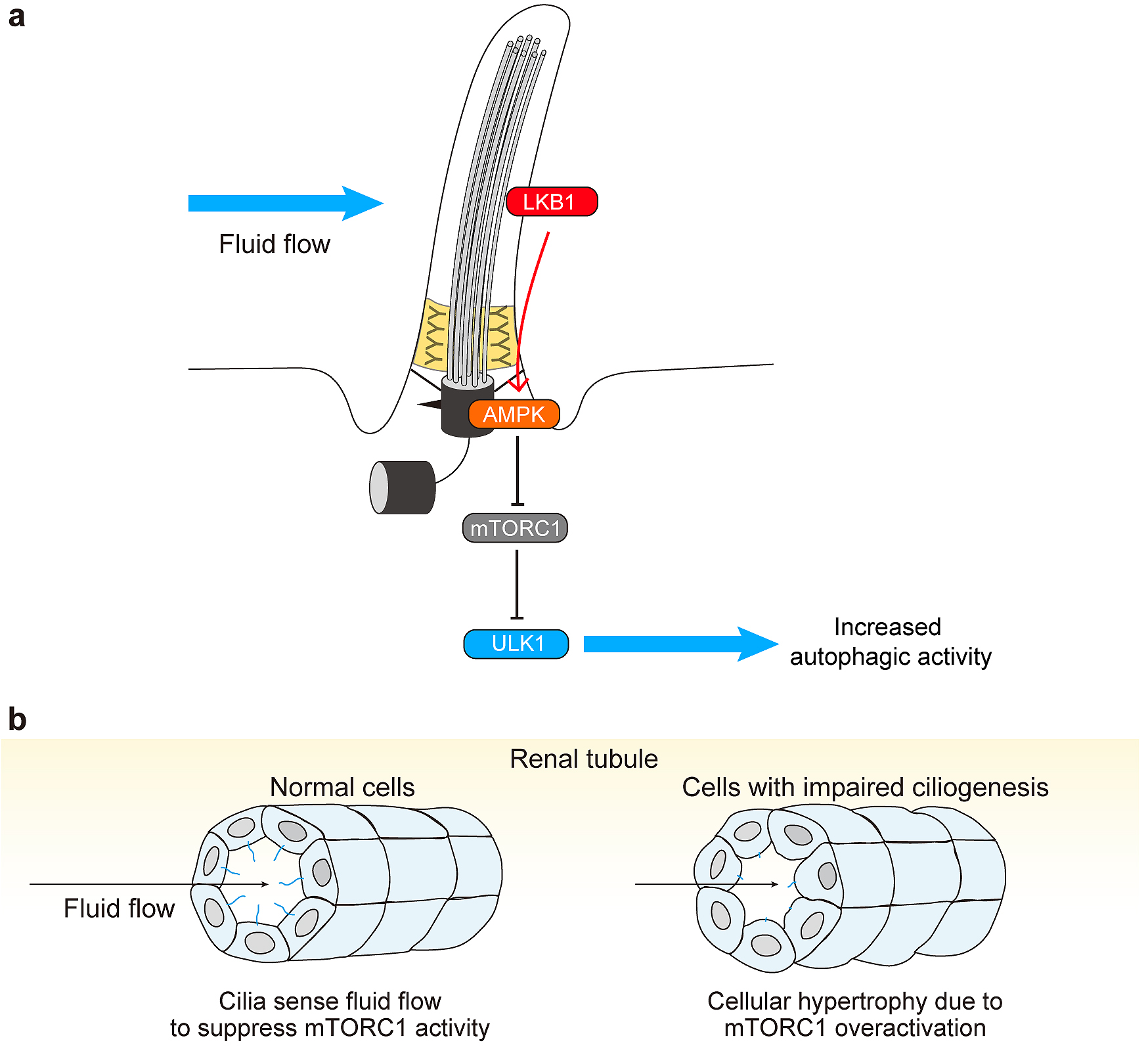
Figure 1. Overview of the autophagy process.
a, In the process of autophagy, the isolation membrane expands and engulfs cytoplasmic components. It finally closes to become an autophagosome. The outer autophagosomal membrane fuses with lysosomes to degrade the sequestered materials.
b, Various types of selective autophagy. Selective cargo containing LIRs is recognized by LC3 and GABARAP subfamily proteins on the autophagosomal membrane. Alternatively, autophagy adaptor proteins mediate interaction between cargo and autophagosomes. Examples of selective cargo include certain proteins and protein aggregates (left), organelles such as mitochondria and the endoplasmic reticulum (middle), and intracellular bacteria (right).
From: Autophagy and Ciliogenesis
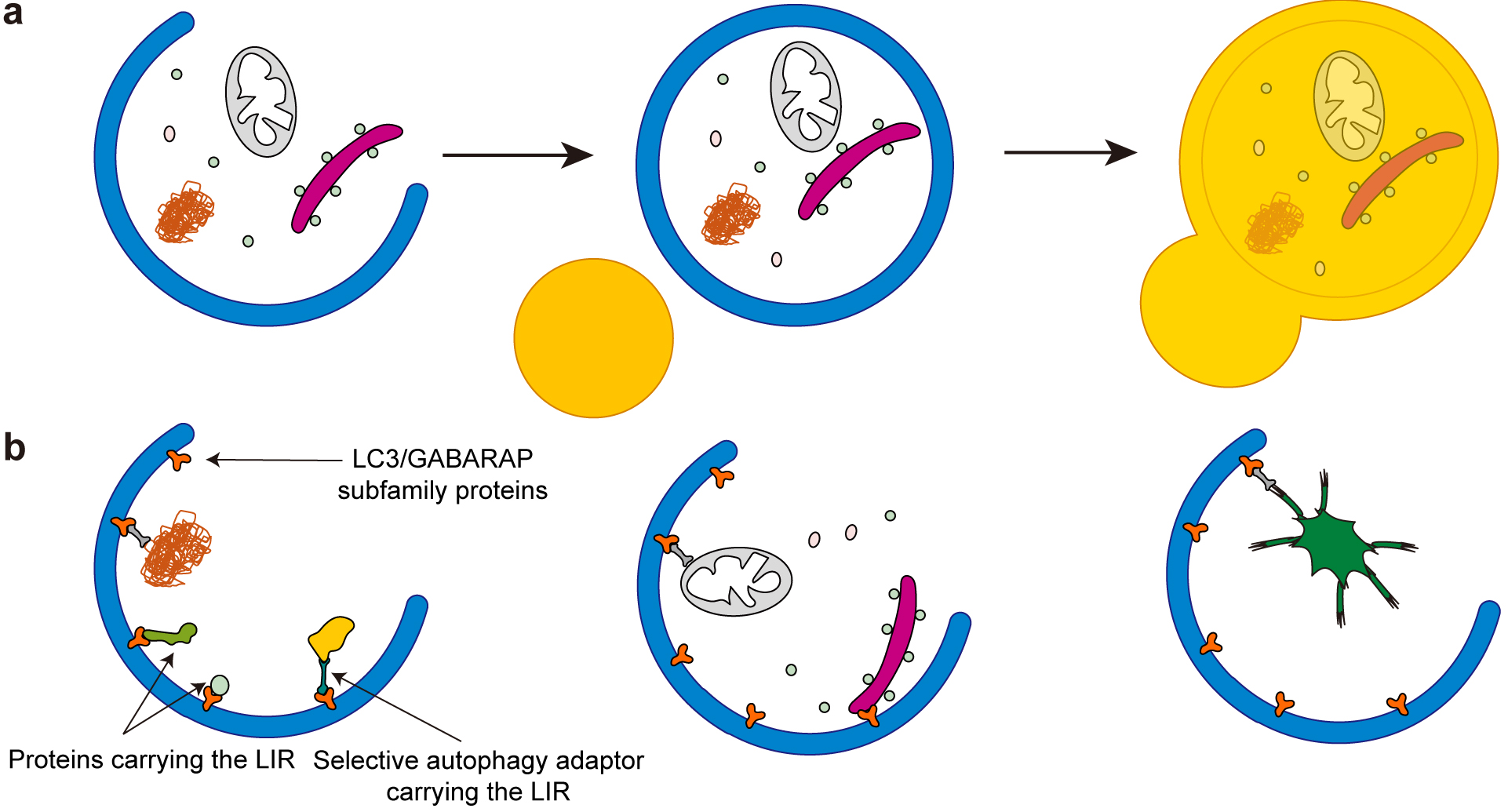
Figure 2. Structure of the primary cilium.
a, The primary cilium is composed of a microtubule-based structure called the axoneme, which is ensheathed in an extension of the plasma membrane called the ciliary membrane. The axoneme of the primary cilium is typically composed of nine doublet microtubules. These microtubules extend from the basal body (mature mother centriole). There is a centrosome, consisting of two centrioles, that is surrounded by centriolar satellites, membraneless organelles that localize and move around the centrosome and cilia. The transition zone functions as a barrier that tightly regulates the entry and exit of proteins. Primary cilia are assembled and maintained by intraflagellar transport (IFT) proteins that are transported along the ciliary microtubules by motor proteins. Various signaling receptors are present in the ciliary membrane to respond to extracellular stimuli.
b, In response to a cilia-forming stimulus such as serum starvation, cells exit the cell cycle and form primary cilia. The basal body migrates toward the plasma membrane and forms the ciliary vesicle, part of which matures to the ciliary membrane. Finally, the basal body docks to the plasma membrane and the axoneme extends to generate the cilium.
From: Autophagy and Ciliogenesis
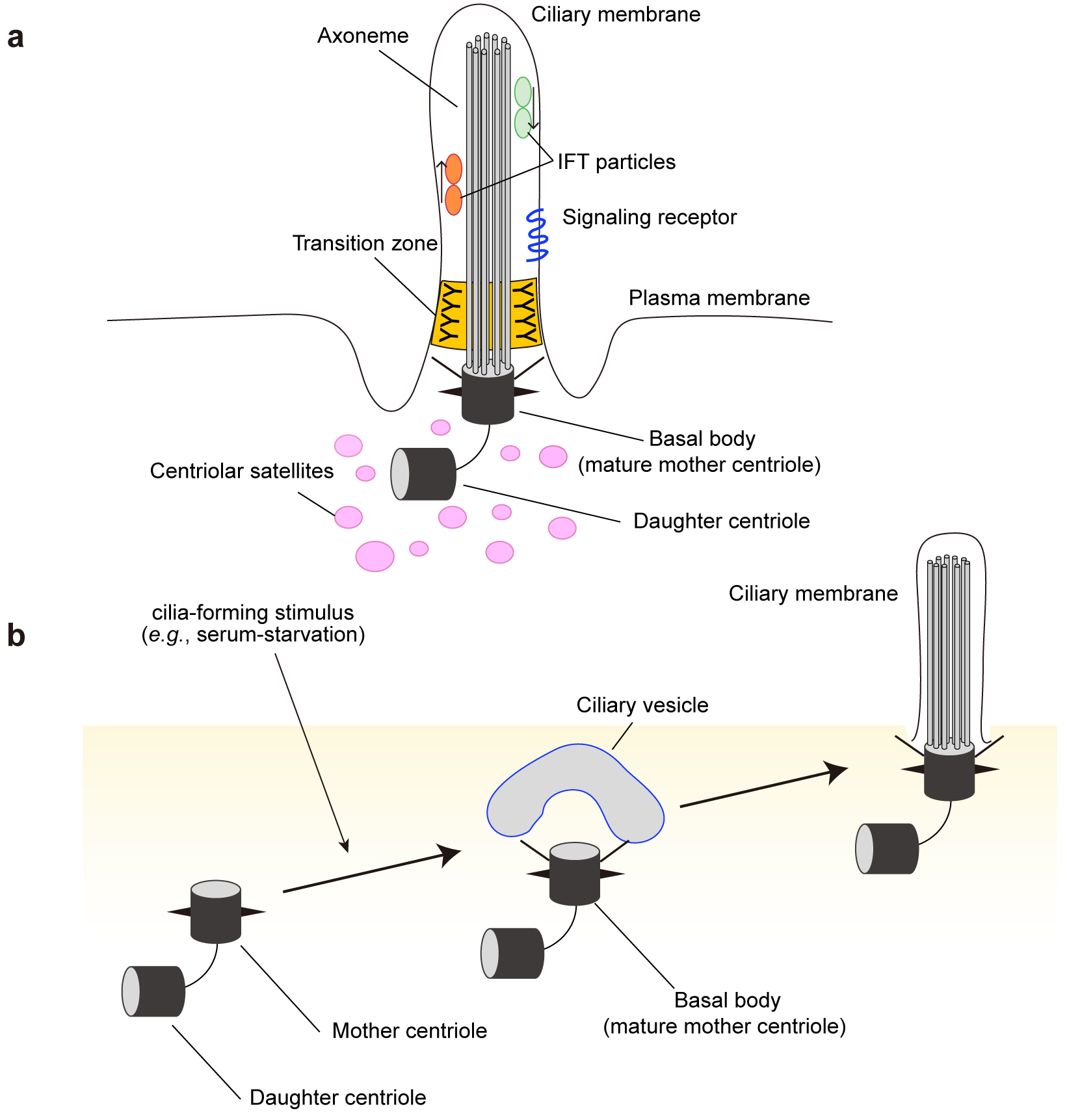
Figure 3. Multi-step regulation of ciliogenesis by autophagy.
In growth conditions, MYH9 stabilizes the intracellular actin filament network and inhibits ciliogenesis. Under cilia-forming starvation conditions, NEK9, as a selective autophagy adaptor, mediates autophagic degradation of MYH9. MYH9 degradation promotes actin network remodeling, which is essential in the early phase of ciliogenesis. Independently, OFD1 at centriolar satellites is degraded by autophagy, which allows the recruitment of cilia-related proteins such as BBS4 toward the periciliary regions. Autophagic degradation of both NEK9-MYH9 and OFD1 is required for efficient ciliogenesis.
From: Autophagy and Ciliogenesis
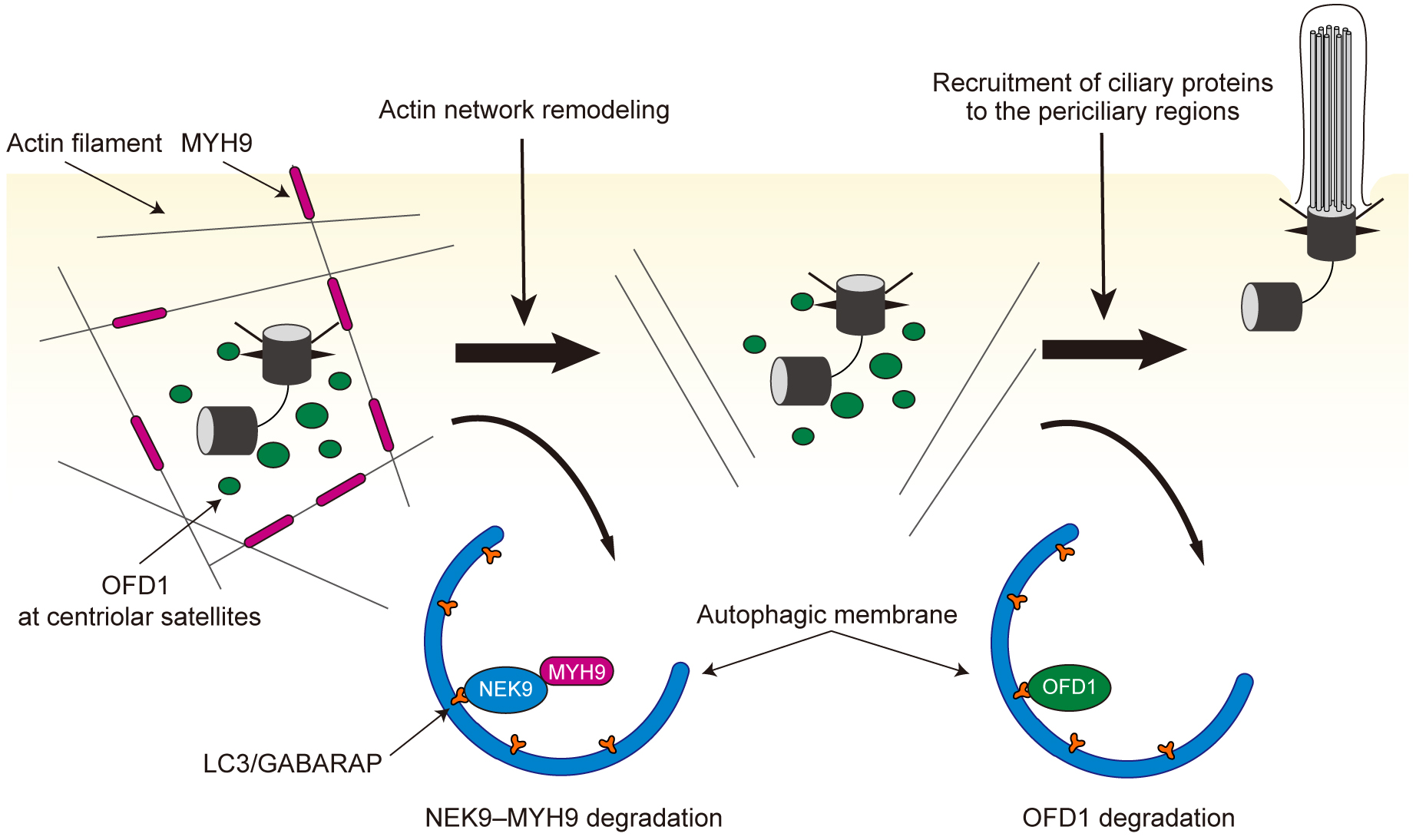
Figure 4. Primary cilia regulate autophagy.
a, Primary cilia sense extracellular shear stress induced by fluid flow and activate liver kinase B1 (LKB1) at the ciliary compartment. LKB1 activates AMP-activated protein kinase (AMPK) at the basal body, which inactivates mTORC1 and activates the ULK1 kinase complex, leading to autophagy upregulation.
b, Normal renal epithelial tubular cells of renal tubules form primary cilia on the lumen side to sense fluid flow (urine) and suppress mTORC1 activity. Cells with defective primary cilia fail to sense fluid flow and show cellular hypertrophy due to mTORC1 overactivation.
From: Autophagy and Ciliogenesis