Corresponding author: Emilie Louise Akiko Matsumoto-Takahashi, etakahashi@slcn.ac.jp
DOI: 10.31662/jmaj.2023-0171
Received: October 27, 2023
Accepted: February 5, 2024
Advance Publication: April 1, 2024
Published: April 15, 2024
Cite this article as:
Das Barshan A, Matsumoto-Takahashi ELA. Efficacy of COVID-19 Vaccines in Patients with Hematological Malignancy Compared to Healthy Controls: A Systematic Review and Meta-analysis. JMA J. 2024;7(2):153-171.
Background: The possibility of developing a severe coronavirus infectious (COVID-19) disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has increased, particularly in patients with hematological malignancies. These patients are more likely to produce less antibody protection due to the immunocompromised nature of the disease and the anticancer treatments. Therefore, the present systematic review intended to evaluate the seroconversion rate of COVID-19 vaccines in patients with hematological malignancies compared with healthy controls.
Methods: A comprehensive systematic search was conducted in Medline via PubMed, EMBASE, and the World Health Organization COVID-19 Research Database, as well as other searches (i.e., reference list from article search and manual searches), from December 2020 to May 2022. The outcome of interest included estimating the seroconversion rates following COVID-19 vaccination in patients with hematological malignancies and comparing them with those in healthy controls. After two-step screening, the data were extracted and the summary measures were calculated using a random-effects model.
Results: A total of 39 articles regarding patients with hematological malignancies were included in the present review. After the first vaccine dose, these patients had considerably lower antibody response rates (37.0%) compared with healthy controls (74.5%). Following the second vaccine dose, the seroconversion rate in patients reached 66.8%, whereas it peaked at 97.9% in the healthy controls following complete immunization. Notably, the BNT162b2 and ChAdOx1 vaccine combination achieved the highest seropositivity rate of approximately 70%. Multiple myeloma, chronic lymphocytic leukemia, and lymphoma were the cancers of interest in most of the studies.
Conclusions: The results of the present study highlighted the comparatively low seropositivity rates in patients with hematological malignancies, with substantial variations in rates across disease groups. The findings emphasize the possibility of additional booster doses for these individuals to enhance their immunity against SARS-CoV-2.
Key words: COVID-19 vaccines, Hematological malignancy, Seroconversion, Immunogenicity, Systematic review
The global spread of COVID-19 caused by SARS-CoV-2 called for an immediate worldwide action to combat its high mortality. The Food and Drug Administration approved an Emergency Use Authorization for the first COVID-19 vaccine on December 11, 2020 (1). Several vaccines were developed in response, including BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), AZD1222 (Oxford/AstraZeneca), Ad26.COV2.S (Johnson & Johnson), Sputnik V (Gamaleya), and BBIBP-CorV (Sinopharm), with efficacy values between 60% and 94%, and were generally well tolerated (2). In the subsequent year, several clinical trials and observational studies assessed the efficacy of the COVID-19 vaccination in healthy individuals. However, few trials included individuals with chronic disease who had relatively safe profiles. Immunocompromised individuals were often excluded from such studies because of vulnerabilities, resulting in a gap in the empirical data for this cohort.
Pertinently, patients with cancer are susceptible to severe COVID-19 infection compared to the general population. An Italian study elucidated that the hospitalization rate for patients with cancer is approximately 56.6%, which is markedly higher than the 34.4% observed in the general population. Furthermore, patients with cancer exhibit a mortality rate of 14.7%, in contrast to 4.5% of healthy individuals (3). A comprehensive meta-analysis covering 52 studies reported a pooled case mortality rate of approximately 25.6% among patients with cancer infected with COVID-19 (4).
Specifically, patients with hematological malignancies, which constitute approximately 9% of all cancer diagnoses, are predisposed to severe SARS-CoV-2 infections because of immune system compromises (5). They have a 37% higher mortality risk when infected with COVID-19 and are prone to prolonged virus shedding and delayed seroconversion (6), (7). Given the aforementioned issues, it is crucial for these individuals to receive the COVID-19 vaccine. However, many clinical trials excluded these patients, leading to limited data on the vaccine efficacy of this group. Studies indicate that they have a reduced response to vaccines, putting them at risk of fatal COVID-19 infection and death (8), (9). Therefore, the Centers for Disease Control and Prevention (CDC) has approved an additional booster dose for all immunocompromised patients (10), (11).
This systematic review compared the vaccine efficacy between healthy adults and patients with hematological malignancies. A systematic review published in early 2022 reported the immunogenicity of seven types of hematological malignancies against the COVID-19 vaccine (12). Although some other studies have evaluated the seroconversion rate in these patients, very few have made direct comparisons with healthy controls (13), (14), (15). This review seeks to fill that knowledge gap by analyzing vaccine responses in patients with hematological malignancies in relation to healthy individuals.
The present systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis checklist 2020 by Page et al. (16). The review protocol was registered with PROSPERO, the prospective international register of systematic reviews (CRD42022342545).
A systematic literature search was conducted using four databases, including Medline via PubMed, EMBASE, Cochrane Library, and World Health Organization (WHO) COVID-19 Research Database. All searches included Medical Subject Headings (MeSH) terms and keywords, which were combined with the Boolean operators AND and OR (Table 1 and 2). No restrictions on the language of publication were applied. Articles from December 2020 to May 2022 were included. The references within these articles were also explored to reach the maximum number of related papers.
Table 1. Population, Intervention, Comparison, Outcome, and Study Types.
| Characteristics | Inclusion | Exclusion |
|---|---|---|
| Population | Adult participants over 18 years with hematological malignancy and had received at least one dose of the COVID-19 vaccine. | Patients with any other diseases and/or any group other than healthy populations. |
| Intervention (Exposure) | First, second, or booster doses of COVID-19 vaccination | Nonvaccinated population |
| Comparison | Immune response to COVID-19 vaccination in patients with hematological malignancies and healthy control group. Immunoglobulin G (IgG) level, Neutralizing antibody (nAb) level | Any malignancy other than patients with hematological malignancies, i.e., solid tumor. |
| Outcome | Rates of seropositivity after one or two doses of COVID-19 vaccine, rates of positive nAb response after one or two doses of vaccine. | Side effects (if any) of the vaccines on the exposed and healthy group, cross-classification of the rates of seropositivity, and rates of positive nAb response with respect to other potential confounders. |
| Study type | All types of observational studies, experimental studies, and clinical trials. | Incomplete studies, studies not reporting any antibody level, and studies reporting CD4 and CD8 cell count in response to COVID-19 vaccination. |
Table 2. Search Strategies.
| Characteristics | Description |
|---|---|
| Databases | Medline via PubMed |
| EMBASE | |
| WHO COVID-19 Research Database | |
| Cochrane Library | |
| Other searches | Manual search using Keywords in Google Scholar |
| Reference list from selected articles | |
| Boolean operators | AND |
| OR | |
| Antibody or seroconversion-related keywords, MeSH terms | “Antibody formation” |
| “Seroconversion” | |
| “Antibodies, neutralizing” | |
| “Neutralizing Antibodies” | |
| “seropositive*” | |
| “seroconversion*” | |
| “Antibody produce*” | |
| “Antibody response*” | |
| “Neutralizing antibody*” | |
| Hematologic malignancy-related keywords, MeSH terms | “Primary Myelofibrosis” |
| “Polycythemia Vera” | |
| “Myelodysplastic Syndromes” | |
| “Waldenstrom Macroglobulinemia” | |
| “Lymphoma” | |
| “Multiple Myeloma” | |
| “Multiple Myeloma” | |
| “Leukemia” | |
| “Leukemia” | |
| “Hematologic Neoplasms” | |
| “Hematologic neoplasm*” | |
| “Hematologic malignan*” | |
| “malignant*” | |
| “neoplasm*” | |
| “hematologic*” | |
| “hematologic*” | |
| COVID-19-related keywords, MeSH terms | “Vaccination” |
| “COVID-19 vaccines/administration and dosage” | |
| “COVID-19 vaccines/immunology” | |
| “SARS-COV-2 VACCINES” | |
| “COVID-19 VACCINES” |
Initially, the title and abstract were carefully checked for preliminary screening. In the second stage of screening, Rayyan QCRI, an intelligent systematic review tool for literature screening developed by Ouzzani and Hammady, was used (17). The inclusion criteria comprise all completed randomized controlled trials, quasi-experimental studies, and observational studies. Studies assessing the efficacy of the COVID-19 vaccine in healthy individuals versus those with hematological malignancies were considered. Selected studies had at least one reported neutralizing antibody defined as seroconversion and the seroconversion rates of both groups after COVID-19 vaccination.
Studies lacking sufficient details on target populations and outcomes or not enabling effect size calculation (e.g., no data on the means and standard deviations for the patient and control groups, respectively) were excluded. The review excluded case reports, review papers, nonacademic publications such as editorials, and conference proceedings. The specific criteria of the population, intervention, comparison, outcomes, and study types are summarized in Table 1.
Two authors (ADB and ELAM-T) conducted an independent screening of the titles and abstracts of relevant papers and assessed the eligibility of full-text articles. Disagreements were resolved through discussion and consensus and were finally checked. Finally, articles for which the full text was inaccessible were eliminated and the records of these excluded articles were maintained in a supplementary document.
Data were extracted independently by two authors (ADB and ELAM-T) using a Microsoft Office spreadsheet. Extracted information included study information (first author, year, country, and study design), population (sample characteristics, time points, hematological malignancy type, mean age in years, and % of male), exposure (vaccine, dose interval, and dose administered), comparison (comparison group, mean age in years, and % of male), and outcome (seroconversion rate and seroconversion cutoff value). A recheck was conducted for any unavailable data from a particular study. Mendeley (reference management software) was used for data management and references.
The findings of this review were presented in a narrative form. The Mantel–Haenszel method was used to estimate the pooled risk ratio and the corresponding 95% confidence interval for the outcome of interest. The hypothesis was tested using the Z statistic (level of significance p < 0.05). Between-study heterogeneity was estimated using Cochran’s Q and I2 indices. The chi-square test was used to test statistical heterogeneity (I2) (18). Random-effects meta-analysis was performed under the appearance of statistical heterogeneity. The between-study variance (τ2) in the random-effects model was estimated using the der Simonian–Laird estimator. The risk ratio (RR) was calculated as a relative effect, along with a 95% confidence interval (CI). All of the results of the meta-analysis were presented in a forest plot. Two different meta-analyses were performed: one for the outcome after the first dose and another for the outcome after the second dose. All analyses were performed using the programing language R (19).
The risk of bias for each study was assessed using the Newcastle–Ottawa scale for cohort studies (20). Each study was classified as low/intermediate/high risk of bias on the basis of the score obtained in three domains, namely, selection, comparability, and outcome domains. The three domains include eight criteria. Scores ≥7–9, 4–6, and < 4 are considered low, intermediate, and high risks of bias study, respectively.
The publication bias of the selected studies was checked using qualitative and quantitative methods. A funnel plot was used as a qualitative tool to check the publication bias through visual inspection. Another quantitative method was used to assess publication bias, that is, Egger’s test (21).
From the initial 2,311 studies, 2,029 came from PubMed; 153, from EMBASE; 37, from the WHO COVID-19 Research Database; and 92, from other sources. Our initial stage of the search included 2,311 studies, of which 2,029 were from PubMed; 153, from EMBASE; 37, from the WHO COVID-19 Research Database; and 92, from other sources, i.e., manual searches and reference lists from literature (Figure 1). After removing 41 duplicates and screening titles, 2,118 articles were excluded. Of the 152 articles assessed in full, 113 were discarded because of not meeting the inclusion criteria. Finally, 39 articles representing approximately 10,854 patients were selected for this systematic review and meta-analysis. The study selection process is detailed in Figure 1.
After the completion of the full screening, 39 studies were selected (8), (22), (23), (24), (25), (26), (27), (28), (29), (30), (31), (32), (33), (34), (35), (36), (37), (38), (39), (40), (41), (42), (43), (44), (45), (46), (47), (48), (49), (50), (51), (52), (53), (54), (55), (56), (57), (58), (59) for our final analysis. Most of the studies were prospective cohort studies that included a comparison between seroconversion rates in both the patient group and control group (Table 3). Different studies used different cutoff values for seropositivity. The population characteristics of all studies are included in Table 3.
Furthermore, various types of hematological malignancies were addressed by the different studies. Multiple myeloma and chronic lymphocytic leukemia were the most concerning malignancies evaluated by 12 and 10 studies, respectively. Of the 39 studies, 8 were conducted in the USA; 6, in the UK; 5, in Italy; 7, in Israel; 5, in Greece; 3, in France; 2, in Belgium; and 1 each, in Austria, Australia, and Sweden. In addition, 34 out of the 39 studies were published in 2021, and the rest in 2022.
Data on the proportion of male and female patients had been compiled from 38 studies. However, data on the gender distribution in the control group were available from only 20 studies. There were 45% females and 55% males in the patient group. By contrast, 58% of females and 42% of males were identified in the control group.
Of the 39 studies, 17 assessed the immunogenicity of both BNT162b2 and mRNA-1273; 11 assessed the immunogenicity of BNT162b2; 7 assessed the immunogenicity of both BNT162b2 and AZD1222; 2 assessed the immunogenicity of BNT162b2, mRNA-1273, and Ad26.COV2. S; and 2 assessed the immunogenicity of BNT162b2 and ChAdOx1, BNT162b2, mRNA-1273, and AZD1222.
Among the 39 studies, 14 focused on the seroconversion rate after the first dose and 35 included information on the second dose. Ten studies reported seroconversion after both the first and second doses. This review represents the seroconversion rates according to the type and dose of the vaccine. The BNT162b2 and ChAdOx1 vaccine combination resulted in the highest seropositivity, peaking rate at approximately 70% after the completion of both doses. However, BNT162b2 and mRNA-1273 also achieved similar seropositivity rates.
Multiple myeloma, chronic lymphocytic leukemia, and lymphoma were the predominant concerns among those studies. In total, 15 studies reported seropositivity among patients with multiple myeloma, which was substantially lower than that of the healthy controls. Among these patients, the highest seroconversion rate was 89% after the second dose. A total of 10 studies provided information on CLL, whereas 12 studies discussed lymphoma. The seropositivity rates reported in each study included in this systematic review are shown in Table 3. The highest seropositivity rates in CLL and lymphoma were 75% and 71%, respectively, which were less than the seroconversion rates achieved by patients with multiple myeloma. Conversely, several studies on these malignancies reported 98%–100% seropositivity among control groups.
Table 3. Population Characteristics of the Included Studies.
| Study information | Population | Exposure | Comparison | Outcome | Risk of bias | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Author, Year, Country, Study design | Seroconversion in patients’ group (S.R-Seroconversion rate) | Hematological malignancy type | Age in years, % of male | Vaccine | Dose interval | Dose administered | Comparison group, age in years, % of male | Seroconversion in healthy controls (S.R-Seroconversion rate) | Seroconversion cutoff value | |
| Aleman et al., 2021, USA, Prospective cohort | Dose 2: | MM | Mean [range]: 65 [47–79], 63% | BNT162b2, mRNA-1273 | 28D or 21D | Second Dose | Healthy participants (n = 12), Mean [range]: 59 [45–64], 50% | 12 | ≥3.2 AU/mL | 7 |
| Patients (n = 44) | (n = 12) | |||||||||
| Seronegative (n = 27) | S.R-100% | |||||||||
| Seropositive (n = 17) | ||||||||||
| S.R-39% | ||||||||||
| Avivi et al., 2021, Israel, Prospective cohort | Dose 2: | MM, SM | Median [range]: 70 [38–94], 56% | BNT162b2, mRNA-1273 | 21D | Second Dose | Median [range]: 67 [41–84], 42.2% | 63 | ≥0.8 IU/mL | 7 |
| Patients (n = 171) | (n = 64) | |||||||||
| Seronegative (n = 38) | S.R-98% | |||||||||
| Seropositive (n = 133) | ||||||||||
| S.R-78% | ||||||||||
| Bergman et al., 2021, Sweden, Prospective cohort | Dose 2: | CLL | NA | BNT162b2, mRNA-1273 | 21D | Second Dose | NA | 69 | ≥0.8 U/mL | 9 |
| Patients (n = 388) | (n = 2) | |||||||||
| Seronegative (n = 108) | S.R-95.8% | |||||||||
| Seropositive (n = 280) | ||||||||||
| S.R-72% | ||||||||||
| Bitoun et al., 2021, France, Retrospective cohort | Dose 2: | MM | Median [range]: 71 [46–93], 44% | BNT162b2, mRNA-1273 | 28D | Second Dose | Median [range]: 58 [26–88], 28.6% | 26 | ≥0.4 IU/mL | 5 |
| Patients (n = 27) | (n = 28) | |||||||||
| Seronegative (n = 3) | S.R-96% | |||||||||
| Seropositive (n = 24) | ||||||||||
| S.R-89% | ||||||||||
| Canti et al., 2021, Belgium, Prospective cohort | Dose 2: | alloHCT | Median [range]: 60 [26–76], 47.5% | BNT162b2, mRNA-1273 | 21D | Second Dose | Median [range]: 48 [23–64], 27.5% | 40 | ≥5 IU/mL | 8 |
| Patients (n = 37) | S.R-88% | |||||||||
| Seronegative (n = 5) | ||||||||||
| Seropositive (n = 32) | ||||||||||
| S.R-86% | ||||||||||
| Chowdhury et al., 2021, UK, Retrospective cohort | Dose 1: | CML, ET, PV, MF, MDS | Median [IQR]: 62 [52–73], 45.8% | BNT162b2, AZD1222 | 2 W | First Dose | Median [range]: 62 [60–76] | 224 | ≥50 AU/mL | 6 |
| Patients (n = 59) | (n = 232) | |||||||||
| Seronegative (n = 25) | S.R-97% | |||||||||
| Seropositive (n = 34) | ||||||||||
| S.R-58% | ||||||||||
| Chung et al., 2021, USA, Prospective cohort | Dose 1: | leukemia, lymphoma, MM | Median [range]: 65 [22–97], 56.4% | BNT162b2, mRNA-1273 | 28D or 21D | First Dose and Second Dose | Median [range]: 31 [22–67] | First Dose: 59 | Immunoassay ≥50.0 AU/mL | 6 |
| Patients (n = 167) | (n = 59) | |||||||||
| Seronegative (n = 81) | S.R-100% | |||||||||
| Seropositive (n = 86) | Second Dose: 54 | |||||||||
| (n = 54) | ||||||||||
| Dose 2: | S.R-100% | |||||||||
| Patients (n = 456) | ||||||||||
| Seronegative (n = 142) | ||||||||||
| Seropositive (n = 314) | ||||||||||
| S.R-51% & 69% | ||||||||||
| Crombie et al., 2021, USA, Prospective cohort | Dose 1: | Lymphoma and CLL | Median [range]: 69 [30–82], 43.5% | BNT162b2, mRNA-1273 | 28D or 21D | First Dose and Second Dose | Median [range]: 24 [22–56], 43.5% | First Dose: 23 | 1.07 | 5 |
| Patients (n = 22) | Second Dose: 23 | |||||||||
| Seronegative (n = 13) | ||||||||||
| Seropositive (n = 9) | ||||||||||
| Dose 2: | ||||||||||
| Patients (n = 21) | ||||||||||
| Seronegative (n = 9) | ||||||||||
| Seropositive (n = 12) | ||||||||||
| S.R-41% & 57% | ||||||||||
| Fiorino et al., 2021, Italy, Prospective cohort | Dose 2: | MF | Median [range]: 67 [31–85], 50% | BNT162b2, mRNA-1273 | 3–4 W | Second Dose | NA | 40 | ≥30% | 7 |
| Patients (n = 42) | (n = 40) | |||||||||
| Seronegative (n = 10) | S.R-100% | |||||||||
| Seropositive (n = 32) | ||||||||||
| S.R-76% | ||||||||||
| Gastinne et al., 2022, France, Prospective cohort | Dose 2: | lymphoma, leukemia | Median [range]: 62 [21–79], 60% | BNT162b2 | 28D | Second Dose | NA | 25 | ≥0.8 U/mL | 6 |
| Patients (n = 20) | ||||||||||
| Seronegative (n = 14) | ||||||||||
| Seropositive (n = 6) | ||||||||||
| S.R-30% | ||||||||||
| Gavriatopoulou et al., 2021, Greece, Prospective cohort | Dose 1: | WM, CLL, NHL | Median [IQR]: 75 [40–88], 48.3% | BNT162b2, AZD1222 | 21D-3M | First Dose | NA | 114 | ≥30% = positive, ≥50% = clinically relevant inhibition) | 8 |
| Patients (n = 58) | (n = 232) | |||||||||
| Seronegative (n = 50) | S.R-54% | |||||||||
| Seropositive (n = 8) | ||||||||||
| S.R-14% | ||||||||||
| Gavriatopoulou et al., 2021, Greece, Prospective cohort | Dose 1: | WM | Median [IQR]: 73 [64–81], 43.4% | BNT162b2, AZD1222 | 21D-3M | First Dose and Second Dose | Median [IQR]: 66 [62–82], 46.2% | First Dose: 212 | ≥30% = positive, ≥50% = clinically relevant inhibition) | 8 |
| Patients (n = 106) | Second Dose: 212 | |||||||||
| Seronegative (n = 70) | S.R-96.2% | |||||||||
| Seropositive (n = 36) | ||||||||||
| Dose 2: | ||||||||||
| Patients (n = 74) | ||||||||||
| Seronegative (n = 29) | ||||||||||
| Seropositive (n = 45) | ||||||||||
| S.R-34% & 61% | ||||||||||
| Ghione et al., 2021, USA, Prospective cohort | Dose 2: | lymphoma | Median [range]: 70 [35–91], 52.3% | BNT162b2, mRNA-1273 and Ad26.COV2.S | 28D or 21D | Second Dose | NA | 154 | ≥1.0 | 6 |
| Patients (n = 86) | (n = 154) | |||||||||
| Seronegative (n = 50) | S.R-100% | |||||||||
| Seropositive (n = 36) | ||||||||||
| S.R-42% | ||||||||||
| Guglielmelli et al., 2021, Italy, Prospective cohort | Dose 1: | MF, ET, PV | NA | BNT162b2, AZD1222 | 28D or 21D | First Dose | NA | 14 | ≥15 AU/mL | 6 |
| Patients (n = 30) | (n = 14) | |||||||||
| Seronegative (n = 12) | S.R-100% | |||||||||
| Seropositive (n = 18) | ||||||||||
| S.R-60% | ||||||||||
| Herishanu et al., 2021, Israel, Prospective cohort | Dose 2: | CLL | Median [IQR]: 69.0 [63.0–73.7], 67.1% | BNT162b2 | 21D | Second Dose | Median [IQR]: 68 [64–74.7] | 52 | ≥0.8 IU/mL = positive | 8 |
| Patients (n = 52) | (n = 52) | |||||||||
| Seronegative (n = 25) | S.R-100% | |||||||||
| Seropositive (n = 27) | ||||||||||
| S.R-52% | ||||||||||
| Jurgens et al., 2021, USA, Prospective cohort | Dose 2: | Lymphoma and CLL | Median [range]: 71 [24–90], 53.7% | BNT162b2, mRNA-1273 | 28D or 21D | Second Dose | NA | 35 | 10000 | 5 |
| Patients (n = 67) | ||||||||||
| Seronegative (n = 36) | ||||||||||
| Seropositive (n = 31) | ||||||||||
| S.R-46% | ||||||||||
| Lim et al., 2021, UK, Prospective cohort | Dose 1: | lymphoma | Median [IQR]: 69 [57–74], 62.8% | ChAdOx1, BNT162b2 | 10-12 W | First Dose and Second Dose | Median [IQR]: 45 [34–47], 33.3% | 150 | Meso scale discovery >0.55 BAU/mL, Anti–SARS-CoV-2 RBD IgG >0.73 BAU/mL | 6 |
| Patients (n = 59) | (n = 150) | |||||||||
| Seronegative (n = 27) | S.R-100% | |||||||||
| Seropositive (n = 32) | ||||||||||
| Dose 2: | ||||||||||
| Patients (n = 86) | ||||||||||
| Seronegative (n = 25) | ||||||||||
| Seropositive (n = 61) | ||||||||||
| S.R-54% & 71% | ||||||||||
| Mairhofer et al., 2021, Austria, Prospective cohort | Dose 2: | Cancer | NA | BNT162b2, mRNA-1273 | 28D or 21D | Second Dose | NA | 28 | ≥50 IU/mL | 7 |
| Patients (n = 45) | (n = 29) | |||||||||
| Seronegative (n = 19) | S.R-96.6% | |||||||||
| Seropositive (n = 26) | ||||||||||
| S.R-58% | ||||||||||
| Malard et al., 2021, France, Retrospective cohort | Dose 2: | LM, MM | Median [range]: 68.9 [21.5–91.7], 59.7% | BNT162b2 | 28D | Second Dose | NA | ≥50 AU/mL = positive; ≥3100 = neutralization) | 9 | |
| Patients (n = 196) | ||||||||||
| Seronegative (n = 105) | ||||||||||
| Seropositive (n = 91) | ||||||||||
| S.R-46% | ||||||||||
| Marasco et al., 2022, Italy, Prospective cohort | Dose 2: | LM | Median [range]: 56 [46–62], 56.3% | BNT162b2, mRNA-1273 | 28D or 21D | Second Dose | Median [range]: 56 [46–62], 56.3% | 167 | ≥0.8 IU/mL | 9 |
| Patients (n = 167) | (n = 167) | |||||||||
| Seronegative (n = 60) | S.R-100% | |||||||||
| Seropositive (n = 107) | ||||||||||
| S.R-64% | ||||||||||
| Marchesi et al., 2022, Italy, Prospective cohort | Dose 2: | MM | NA | BNT162b2 | 21D | Second Dose | NA | 28 | ≥15 U/m | 6 |
| Patients (n = 49) | (n = 28) | |||||||||
| Seronegative (n = 12) | S.R-100% | |||||||||
| Seropositive (n = 37) | ||||||||||
| S.R-76% | ||||||||||
| McKenzie et al., 2021, UK, Prospective cohort | Dose 2: | HM | Median [IQR]: 35 [27–48], 64.7% | BNT162b2, mRNA-1273 | 70D | Second Dose | Median [IQR]: 66 [52.75–73], 69.2% | 22 | antibody level ED50, nAb assay: IC50 | 5 |
| Patients (n = 51) | (n = 26) | |||||||||
| Seronegative (n = 29) | S.R-88% | |||||||||
| Seropositive (n = 22) | ||||||||||
| S.R-43% | ||||||||||
| Monin et al., 2021, UK, Prospective cohort | Dose 1: | Hematological Cancer | Median [IQR]: 73 [64.5–79.5], 52.3% | BNT162b2 | 21D | First Dose and Second Dose | Median [IQR]: 40.5 [31.3–50], 52.9% | First Dose: 32 | ≥70 EC50 = positive | 7 |
| Patients (n = 44) | (n = 34) | |||||||||
| Seronegative (n = 36) | S.R-94% | |||||||||
| Seropositive (n = 8) | Second Dose: 12 | |||||||||
| (n = 12) | ||||||||||
| Dose 2: | S.R-100% | |||||||||
| Patients (n = 5) | ||||||||||
| Seronegative (n = 2) | ||||||||||
| Seropositive (n = 3) | ||||||||||
| S.R-18% & 60% | ||||||||||
| Parry et al., 2022, UK, Prospective cohort | Dose 2: | B cell CLL | Median [IQR]: 67 [60–72], 53% | AZD1222, BNT162b2 | 3 W | Second Dose | Median [IQR]: 71 [64–77], 55.9% | 93 | ≥0.8 | 7 |
| Patients (n = 500) | (n = 93) | |||||||||
| Seronegative (n = 165) | S.R-100% | |||||||||
| Seropositive (n = 335) | ||||||||||
| S.R-67% | ||||||||||
| Parry et al., 2021, UK, Prospective cohort | Dose 1: | CLL | Median [IQR]: 69 [63–74], 54.7% | AZD1222, BNT162b2 | 12 W and 3 W | First Dose and Second Dose | NA | First Dose: 95 | ≥0.8 | 7 |
| Patients (n = 86) | Second Dose: 59 | |||||||||
| Seronegative (n = 57) | (N = 95 & 59) | |||||||||
| Seropositive (n = 29) | S.R-100% | |||||||||
| Dose 2: | ||||||||||
| Patients (n = 12) | ||||||||||
| Seronegative (n = 3) | ||||||||||
| Seropositive (n = 9) | ||||||||||
| S.R-34% & 75% | ||||||||||
| Peeters et al., 2021, Belgium, Prospective multicohort | Dose 2: | Cancer | Median [range]: 63 [25–79], 58.5% | BNT162b2 | 21D | Second Dose | NA | Second Dose: 40 | ≥5 IU/mL | 6 |
| Patients (n = 41) | (n = 40) | |||||||||
| Seronegative (n = 29) | S.R-100% | |||||||||
| Seropositive (n = 12) | ||||||||||
| S.R-29% | ||||||||||
| Perry et al., 2021, Israel, Prospective cohort | Dose 2: | B-NHL | Median [range]: 64 [20–92], 59.1% | BNT162b2 | 21D | Second Dose | Median [range]: 66 [25–83], 44.6% | 65 | ≥0.8 IU/mL | 8 |
| Patients (n = 149) | S.R-98.5% | |||||||||
| Seronegative (n = 76) | ||||||||||
| Seropositive (n = 73) | ||||||||||
| S.R-49% | ||||||||||
| Pimpinelli et al., 2021, Italy, Prospective cohort | Dose 1: | MM, MPM | Median [range]: 73 [47–78] | BNT162b2, mRNA-1273 | 21D | First Dose and Second Dose | Median [range]: 81 [79–87], 50% | First Dose: 36 | ≥15 U/m | 9 |
| Patients (n = 92) | Median [range]: 70 [28–80], 53.3% | Second Dose: 36 | ||||||||
| Seronegative (n = 57) | n = 36 | |||||||||
| Seropositive (n = 35) | S.R-100% | |||||||||
| Dose 2: | ||||||||||
| Patients (n = 92) | ||||||||||
| Seronegative (n = 15) | ||||||||||
| Seropositive (n = 77) | ||||||||||
| S.R-38% & 84% | ||||||||||
| Rahav et al., 2021, Israel, Prospective cohort | Dose 2: | CLL, NHL, MM, and MDS | Median [IQR]: 69 [61–74] | BNT162b2 | 21D | Second Dose | NA | 269 | ≥1.1 | 9 |
| Patients (n = 529) | Median [IQR]: 66 [59–73] | (n = 272) | ||||||||
| Seronegative (n = 175) | Median [IQR]: 62 [49–70] | S.R-98.9% | ||||||||
| Seropositive (n = 354) | Median [IQR]: 73 [66–80], 58.8% | |||||||||
| S.R-67% | ||||||||||
| Schiller-Salton et al., 2021, Israel, Prospective cohort | Dose 2: | MM | Mean ± SD: 67.8 ± 9.9, 58.50% | BNT162b2 | 30D | Second Dose | NA | Second Dose: 360 | ≥150 AU/mL | 8 |
| Patients (n = 176) | ||||||||||
| Seronegative (n = 47) | ||||||||||
| Seropositive (n = 129) | ||||||||||
| S.R-73% | ||||||||||
| Shem-Tov et al., 2022, Israel, Prospective cohort | Dose 2: | HSCT | Mean ± SD: 58 ± 14.0, 63.20% | BNT162b2, mRNA-1273 | 21D | Second Dose | Mean ± SD: 55.6 ± 14.2, 24.3% | 269 | Cut point: >1.1 | 7 |
| Patients (n = 152) | (n = 272) | |||||||||
| Seronegative (n = 34) | S.R-98.9% | |||||||||
| Seropositive (n = 118) | ||||||||||
| S.R-78% | ||||||||||
| Shen et al., 2021, Australia, Prospective cohort | Dose 2: | CLL | Median [range]: 71.5 [22–94], 56.3% | BNT162b2, mRNA-1273, AZD1222 | 2-4 W | Second Dose | NA | 25 | ≥50 AU/mL | 6 |
| Patients (n = 160) | ||||||||||
| Seronegative (n = 72) | ||||||||||
| Seropositive (n = 88) | ||||||||||
| S.R-55% | ||||||||||
| Stampfer et al., 2021, USA, Retrospective cohort | Dose 1: | MM | Median [range]: 68 [35–88], 59.4% | BNT162b2, mRNA-1273 | 28D or 21D | First Dose and Second Dose | Median [range]: 61 [26–85] | First Dose: 31 | 50-250 IU/mL partial response, >250 IU/mL | 8 |
| Patients (n = 96) | Second Dose: 31 | |||||||||
| Seronegative (n = 76) | n-31 | |||||||||
| Seropositive (n = 20) | ||||||||||
| S.R-100% | ||||||||||
| Dose 2: | ||||||||||
| Patients (n = 96) | ||||||||||
| Seronegative (n = 32) | ||||||||||
| Seropositive (n = 64) | ||||||||||
| S.R-21% & 67% | ||||||||||
| Terpos et al., 2021, Greece, Prospective cohort | Dose 1: | CLL, NHL, HL | Mean ± SD: 64.6 ± 14.3, 50% | BNT162b2 | 21D | First Dose and Second Dose | Mean ± SD: 69.8 ± 12.5, 44.9% | First Dose: 152 | GenScript (≥30% = positive, ≥30% = clinically relevant) | 6 |
| Patients (n = 132) | (n = 214) | |||||||||
| Seronegative (n = 103) | S.R-71% | |||||||||
| Seropositive (n = 29) | Second Dose: 214 | |||||||||
| (n = 214) | ||||||||||
| Dose 2: | S.R-98.1% | |||||||||
| Patients (n = 132) | ||||||||||
| Seronegative (n = 65) | ||||||||||
| Seropositive (n = 67) | ||||||||||
| S.R-22% & 51% | ||||||||||
| Terpos et al., 2021, Greece, Prospective cohort | Dose 1: | MM, SM, MGUS | Median [IQR]: 74 [62–80], 54.7% | BNT162b2, AZD1222 | 21D-3M | First Dose and Second Dose | NA | First Dose: 145 | GenScript (≥30% = positive, ≥30% = clinically relevant) | 8 |
| Patients (n = 276) | ||||||||||
| Seronegative (n = 159) | (n = 225) | |||||||||
| Seropositive (n = 117) | ||||||||||
| S.R-64.2% | ||||||||||
| Dose 2: | Second Dose: 204 | |||||||||
| Patients (n = 276) | (n = 226) | |||||||||
| Seronegative (n = 80) | S.R-90.3% | |||||||||
| Seropositive (n = 196) | ||||||||||
| S.R-42% & 71% | ||||||||||
| Terpos et al., 2021, Greece, Prospective cohort | Dose 1: | MM | Mean ± SD: 81.68 ± 7.63, 60.40% | BNT162b2 | 21D | First Dose | Mean ± SD: 81.56 ± 8.11, 54.8% | 57 | GenScript (≥30% = positive, ≥30% = clinically relevant) | 8 |
| Patients (n = 48) | (n = 104) | |||||||||
| Seronegative (n = 36) | S.R-54.8% | |||||||||
| Seropositive (n = 12) | ||||||||||
| S.R-25% | ||||||||||
Table 3. Population Characteristics of the Included Studies.
| Study information | Population | Exposure | Comparison | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Author, Year, Country, Study design | Seroconversion in patients’ group (S.R-Seroconversion rate) | Hematological malignancy type | Age in years, % of male | Vaccine | Dose interval | Dose administered | Comparison group, age in years, % of male | Seroconversion in healthy controls (S.R-Seroconversion rate) | Seroconversion cutoff value | Risk of bias |
| Thakkar et al., 2021, USA, Retrospective cohort | Dose 2: | HM | Median [range]: 67 [27–90], 42% | BNT162b2, mRNA-1273, Ad26.COV2.S | 21D or 28D | Second Dose | NA | 26 | ≥ 50 AU/mL | 7 |
| Patients (n = 66) | (n = 26) | |||||||||
| Seronegative (n = 10) | S.R-100% | |||||||||
| Seropositive (n = 56) | ||||||||||
| S.R-85% | ||||||||||
| Tzarfati et al., 2021, Israel, Prospective cohort | Dose 2: | HM | Median [IQR]: 70 [61–78], 55.9% | BNT162b2 | 21D | Second Dose | Median [IQR]: 69 [58–74], 43.5% | 107 | ≥12 AU/mL positive) | 8 |
| Patients (n = 315) | (n = 108) | |||||||||
| Seronegative (n = 80) | S.R-99% | |||||||||
| Seropositive (n = 235) | ||||||||||
| S.R-75% | ||||||||||
| Van Oekelen et al., 2021, USA, Retrospective cohort | Dose 2: | MM | Median [range]: 68 [38–93], 58.1% | BNT162b2, mRNA-1273 | 28D or 21D | Second Dose | NA | 67 | 5 AU/mL | 8 |
| (n = 260) | (n = 67) | |||||||||
| Seronegative (n = 41) | S.R-100% | |||||||||
| Seropositive (n = 219) | ||||||||||
| 84% | ||||||||||
It was found that there was significant heterogeneity in the meta-analysis of the effects of COVID-19 vaccination in patients with hematological malignancies for the first dose (I2 = 80.6%, p < 0.0001) and for the second dose (I2 = 87.8%, p < 0.0001). Between-study heterogeneity (τ2) for the first vaccine dose was (0.0844, p < 0.01). In the case of the second vaccine dose, between-study heterogeneity (τ2) was (0.0224, p < 0.01).
The RR for seroconversion rate after the first vaccine dose in patients with hematological malignancies compared with healthy controls is presented in Figure 2. There were 14 studies reporting seroconversion after the first vaccine dose in patients with hematological malignancies (n = 1275) compared with healthy controls (n = 1558). The seroconversion rates in patients with hematological malignancies (37.0%) were reduced compared with those in healthy controls (74.5%) after the first vaccine doses (RR: 0.45, CI (0.37, 0.54), Z = −8.78, p < 0.0001).
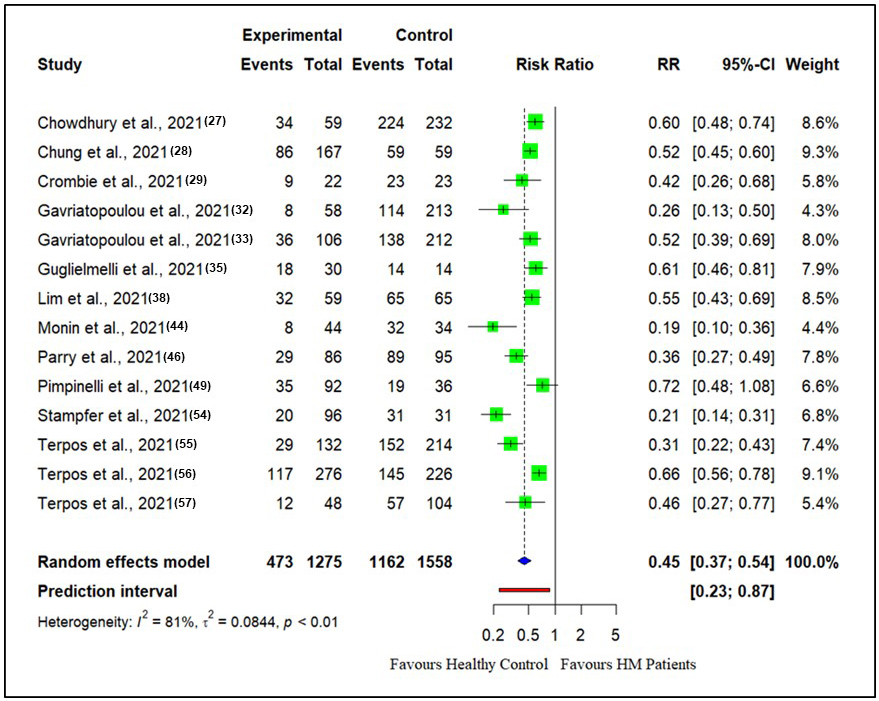
Similarly, after the second vaccine dose, the seroconversion rates for patients with hematological malignancies compared with healthy controls are presented in Figure 3.
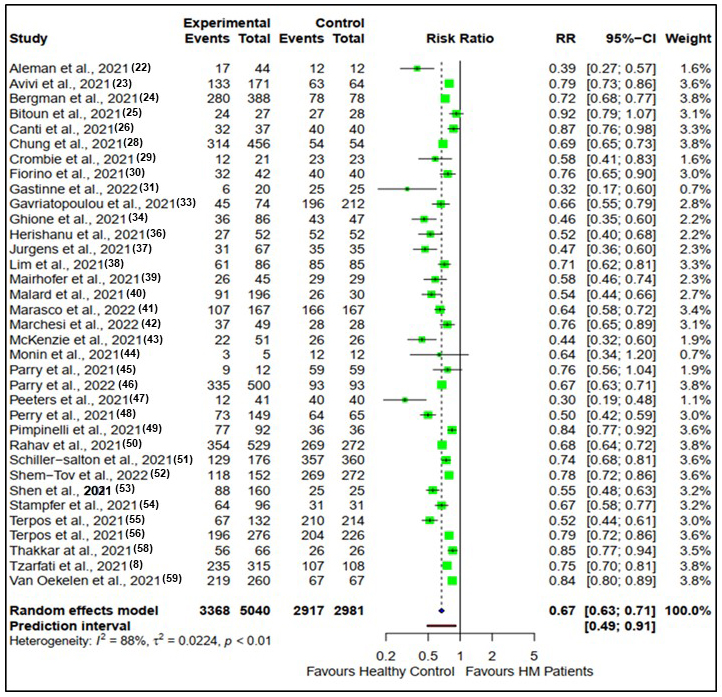
There were 35 studies reporting seroconversion after the second vaccine dose in patients with hematological malignancies (n = 5040) compared with healthy controls (n = 2981). Seroconversion rates in patients with hematological malignancies (66.9%) were reduced compared with healthy controls (97.9%) after the second vaccine doses (RR: 0.67, CI (0.63, 0.71), Z = −13.56, p < 0.0001).
As shown in Table 4, 25 studies were assessed to be at a low risk of bias and 14 at a moderate risk of bias. The risk of bias mainly represented the exposed cohort, with controls not being age-matched, lack of outcome information at the beginning, lack of follow-up length and adequacy of follow-up, and lack of available data at predetermined endpoints. The study list of the Newcastle–Ottawa scale for risk of bias assessment is presented in Table 4.
Table 4. Newcastle–Ottawa Scale for Risk of Bias Assessment of the Included Studies.
| Study | Selection | Comparability | Outcome | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Outcome not present at start | Assessment of outcome | Adequate follow-up length | Adequacy of follow up | |||
| Aleman et al., 2021 (22) | * | * | * | * | * | * | * | 7 | |
| Avivi et al., 2021 (23) | * | * | * | * | * | * | * | 7 | |
| Bergman et al., 2021 (24) | * | * | * | * | ** | * | * | * | 9 |
| Bitoun et al., 2021 (25) | * | * | * | * | * | 5 | |||
| Canti et al., 2021 (26) | * | * | * | * | * | * | * | * | 8 |
| Chowdhury et al., 2021 (27) | * | * | * | * | * | * | 6 | ||
| Chung et al., 2021 (28) | * | * | * | * | * | * | 6 | ||
| Crombie et al., 2021 (29) | * | * | * | * | * | 5 | |||
| Fiorino et al., 2021 (30) | * | * | ** | * | * | * | 7 | ||
| Gastinne et al., 2022 (31) | * | * | * | * | * | * | 6 | ||
| Gavriatopoulou et al., 2021 (32) | * | * | * | * | * | * | * | * | 8 |
| Gavriatopoulou et al., 2021 (33) | * | * | * | * | * | * | * | * | 8 |
| Ghione et al., 2021 (34) | * | * | * | * | * | * | 6 | ||
| Guglielmelli et al., 2021 (35) | * | * | * | * | * | * | 6 | ||
| Herishanu et al., 2021 (36) | * | * | * | ** | * | * | * | 8 | |
| Jurgens et al., 2021 (37) | * | * | * | * | * | 5 | |||
| Lim et al., 2021 (38) | * | * | * | * | * | * | 6 | ||
| Mairhofer et al., 2021 (39) | * | * | * | * | * | * | * | 7 | |
| Malard et al., 2021 (40) | * | * | * | * | ** | * | * | * | 9 |
| Marasco et al., 2022 (41) | * | * | * | * | ** | * | * | * | 9 |
| Marchesi et al., 2022 (42) | * | * | * | * | * | * | 6 | ||
| McKenzie et al., 2021 (43) | * | * | * | * | * | 5 | |||
| Monin et al., 2021 (44) | * | * | * | * | * | * | * | 7 | |
| Parry et al., 2022 (45) | * | * | * | * | * | * | * | 7 | |
| Parry et al., 2021 (46) | * | * | * | * | * | * | * | 7 | |
| Peeters et al., 2021 (47) | * | * | * | * | * | * | 6 | ||
| Perry et al., 2021 (48) | * | * | * | ** | * | * | * | 8 | |
| Pimpinelli et al., 2021 (49) | * | * | * | * | ** | * | * | * | 9 |
| Rahav et al., 2021 (50) | * | * | * | * | ** | * | * | * | 9 |
| Schiller-Salton et al., 2021 (51) | * | * | * | ** | * | * | * | 8 | |
| Shem-Tov et al., 2022 (52) | * | * | ** | * | * | * | 7 | ||
| Shen et al., 2021 (53) | * | * | * | * | * | * | 6 | ||
| Stampfer et al., 2021 (54) | * | * | * | ** | * | * | * | 8 | |
| Terpos et al., 2021 (55) | * | * | * | * | * | * | * | * | 8 |
| Terpos et al., 2021 (56) | * | * | * | * | * | * | * | * | 8 |
| Terpos et al., 2021 (57) | * | * | * | * | * | * | 6 | ||
| Thakkar et al., 2021 (58) | * | * | * | * | * | * | * | 7 | |
| Tzarfati et al., 2021 (8) | * | * | * | ** | * | * | * | 8 | |
| Van Oekelen et al., 2021 (59) | * | * | * | ** | * | * | * | 8 | |
The results of publication bias assessment using a funnel plot are shown in Figure 4. On the basis of this assumption, the funnel plots for the first and second vaccine doses clearly show the presence of publication bias as they are highly asymmetrical.
The present systematic review and meta-analysis using 39 studies included several hematological malignancies for seroconversion. According to the findings of the present study, after the first vaccine dose, patients with cancer had an antibody response rate (37.0%) that was considerably lower than that of the healthy controls (74.5%). Following the second vaccine dose, the seroconversion rate in patients with cancer reached 66.8% whereas it peaked at 97.9% in the healthy controls. This result agreed with the findings of another meta-analysis of 27 studies that revealed seroconversion rates of 37.3%–51% and 57%–60% following the first and second vaccine doses, respectively, in the patient group (12). Patients with multiple myeloma displayed lower seroconversion rates compared with healthy individuals after receiving the COVID-19 vaccine. Out of 2000 patients with myeloma who were vaccinated, seropositivity rates after the first dose varied between 21.43% and 38%. This rate increased to 89% after the second dose. By contrast, the seroconversion rate increased to 89% following the second vaccine dose. However, a previous study found high seroconversion rates of 93% with myeloma therapy and 94% without myeloma therapy after vaccination (60). According to another study, 52% of individuals tested positive for SARS-CoV-2 IgG antibodies following the second vaccine dose, which was lower than that of our result (13). However, two of the studies that we reviewed reported active treatment to be associated with reduced response rates (13), (60). Factors influencing these variable rates in patients with myeloma include the extent of immunoparesis, relapsed cases, monoclonal antibody treatment, and renal failure, which can lead to vaccine inefficacy.
Thirteen studies focused on leukemia, with 10 highlighting the low seropositivity in patients with CLL after vaccination compared with the multiple myeloma group. CLL is less aggressive in stages A and B, requiring intensive treatment mostly in stage C. Because of treatments, especially in stages B and C, patients with CLL often experience relative immune suppression (61). In this review, only 51%–75% of patients with CLL showed an optimal humoral response after COVID-19 vaccination. This aligns with a prior study reporting a 52% seroconversion rate post-immunization (14). One study noted that patients on tyrosine kinase inhibitors had a 16.8% seroconversion rate, which was lower than the 26.8% reported in another systematic review and meta-analysis (62).
There were 12 reviewed studies that reported reduced seroconversion rates in patients with lymphoma. The seropositivity rates after the second dose ranged from 42% to 71%. This finding was consistent with the previous study, which observed an almost 60% seroconversion rate in patients (63). The same study reported a 69% seroconversion rate in patients with allogeneic hematological stem cell transplantation, whereas a considerably high (86.4%) seropositivity rate was found in one of our studies. Regarding vaccine efficacy, a prior study indicated that COVID-19 mRNA vaccines were highly protective in patients with cancer but had a lower efficacy than that of the healthy controls (64).
According to our study, the BNT162b2 and ChAdOx1 vaccine combination as the first and second doses achieved the highest seroprotection (70%) against COVID-19. Almost all vaccines provided a 98%–100% seroconversion rate in healthy controls. Our findings reaffirmed the efficacy of mRNA vaccines (BNT162b2) and viral vector vaccines (ChAdOx1). The mRNA vaccines stimulate helper T cells, producing neutralizing antibodies (nAbs) and developing immune memory, which enhances the protection against COVID-19. This makes BNT162b2 effective even in immunocompromised patients. ChAdOx1, using a nonreplicating adenovirus vector, helps the immune system recognize and combat SARS-CoV-2’s spike protein. This dual-pathway activation benefits patients with hematological malignancies. Taken together, the up-to-date evidence generated by this systematic review and meta-analysis on the antibody response in patients with hematological malignancies and the clinical efficacy of vaccines will aid in formulating a newer vaccine policy for these individuals.
The present review had several limitations. First, only seroconversion data were taken from studies for analysis without any adverse effect of COVID-19 vaccine data. Second, the cutoff points for seropositivity varied across studies. Although it must be considered that the values may differ slightly depending on the testing method or population, it is unlikely that this difference had a significant impact on the comparison between the two groups. Third, most of these studies did not include any prior history of SARS-CoV-2 infection data that might influence the seropositivity analysis. Fourth, the treatment options were not examined, which needs to be analyzed in more detail in the future. Lastly, the immune response to booster doses could not be evaluated because there was not enough information regarding seroconversion by the third and fourth COVID-19 vaccine doses.
The present systematic review clearly illustrated that patients with hematological malignancies have lower antibody titers compared with healthy controls. Therefore, these patients should carefully adhere to COVID-19 preventive measures or should be given priority when receiving a booster dose of the vaccine. Although the response rates were inadequate, vaccination is still regarded as important and should be performed before the start of anticancer therapy whenever possible. Long-term self-protective measures such as mask use, sanitization, and avoidance of social contact are always required for these patients.
None
ADB and ELAM-T conceived the research topic. ADB contributed to the collection of clinical information, data analysis, and manuscript preparation under the supervision of ELAM-T. All authors critically reviewed and revised the manuscript and approved the final version for submission.
This systematic review used studies that are published in several medical databases. Ethics approval was not required for this study.
Mullard A. Pfizer’s COVID-19 vaccine secures first full FDA approval. Nat Rev Drug Discov. 2021;20(10):728.
Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39(8):1091-98.e2.
Rugge M, Zorzi M, Guzzinati S. SARS-CoV-2 infection in the Italian Veneto region: adverse outcomes in patients with cancer. Nat Cancer. 2020;1(8):784-8.
Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43-50.
Keykhaei M, Masinaei M, Mohammadi E, et al. A global, regional, and national survey on burden and Quality of Care Index (QCI) of hematologic malignancies; global burden of disease systematic analysis 1990-2017. Exp Hematol Oncol. 2021;10(1):1-5.
Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737-45.
Kakkassery H, Carpenter E, Patten PEM, et al. Immunogenicity of SARS-CoV-2 vaccines in patients with cancer. Trends Mol Med. 2022;28(12):1082-99.
Herzog Tzarfati K, Gutwein O, Apel A, et al. BNT162b2 COVID‐19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96(10):1195-203.
Agha ME, Blake M, Chilleo C, et al. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect Dis. 2021;8(7):ofab353.
Mair MJ, Berger JM, Mitterer M, et al. Third dose of SARS-CoV-2 vaccination in hemato-oncological patients and health care workers: immune responses and adverse events–a retrospective cohort study. Eur J Cancer. 2022;165:184-94.
CDC. COVID-19 vaccines for people who are moderately or severely immunocompromised [Internet]. 2022 [cited 2024 Oct 18]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html#:~:text=CDC%20recommends%20the%202023%E2%80%932024,2024%20updated%20COVID%2D19%20vaccine.
Teh JSK, Coussement J, Neoh ZCF, et al. Immunogenicity of COVID-19 vaccines in patients with hematologic malignancies: a systematic review and meta-analysis. Blood Adv. 2022;6(7):2014-34.
Bird S, Panopoulou A, Shea RL, et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8(6):e389-92.
Roeker LE, Knorr DA, Thompson MC, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35(9):2703-5.
Narita K, Nakaji S, Tabata R, et al. Antibody response to COVID-19 vaccination in patients with lymphoma. Int J Hematol. 2022;115(5):728-36.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-60.
Ondrikova N, Harris JP, Douglas A, et al. Predicting Norovirus in England using existing and emerging syndromic data: infodemiology study. J Med Internet Res. 2023;25:e37540.
Nguyen LB, Vu LG, Le TT, et al., Impact of interventions on the quality of life of cancer patients: a systematic review and meta-analysis of longitudinal research. Health Qual Life Out. 2023;21(1):1-4.
Egger M, Davey Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-34.
Aleman A, Upadhyaya B, Tuballes K, et al. Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell. 2021;39(11):1442-4.
Avivi I, Balaban R, Shragai T, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol. 2021;195(2):186-93.
Bergman P, Blennow O, Hansson L, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74:103705.
Bitoun S, Henry J, Vauloup-Fellous C, et al. Response to COVID-19 mRNA vaccination in multiple myeloma is conserved but impaired compared to controls. J Hematol Oncol. 2021;14(1):1-66.
Canti L, Humblet-Baron S, Desombere I, et al. Predictors of neutralizing antibody response to BNT162b2 vaccination in allogeneic hematopoietic stem cell transplant recipients. J Hematol Oncol. 2021;14(1):174-0.
Chowdhury O, Bruguier H, Mallett G, et al. Impaired antibody response to COVID‐19 vaccination in patients with chronic myeloid neoplasms. Br J Haematol. 2021;194(6):1010-5.
Chung DJ, Shah GL, Devlin SM, et al. Disease-and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov. 2021;2(6):568-76.
Crombie JL, Sherman AC, Cheng CA, et al. Activity of mRNA COVID-19 vaccines in patients with lymphoid malignancies. Blood Adv. 2021;5(16):3062-5.
Fiorino F, Sicuranza A, Ciabattini A, et al. The slower antibody response in myelofibrosis patients after two doses of mRNA SARS-CoV-2 vaccine calls for a third dose. Biomedicines. 2021;9(10):1480.
Gastinne T, Le Bourgeois A, Coste‐Burel M, et al. Safety and antibody response after one and/or two doses of BNT162b2 anti‐SARS‐CoV‐2 mRNA vaccine in patients treated by CAR T cells therapy. Br J Haematol. 2022;196(2):360-2.
Gavriatopoulou M, Terpos E, Kastritis E, et al. Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine. Clin Exp Med. 2021:1-5.
Gavriatopoulou M, Terpos E, Ntanasis-Stathopoulos I, et al. Poor neutralizing antibody responses in 106 patients with WM after vaccination against SARS-CoV-2: a prospective study. Blood Adv. 2021;5(21):4398-405.
Ghione P, Gu JJ, Attwood K, et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell–directed therapies. Blood. The J Am Soc Hematol. 2021;138(9):811-4.
Guglielmelli P, Mazzoni A, Maggi L, et al. Impaired response to first SARS‐CoV‐2 dose vaccination in myeloproliferative neoplasm patients receiving Ruxolitinib. Am J Hematol. 2021;96(11):E408-10.
Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165-73.
Jurgens EM, Ketas TJ, Zhao Z, et al. Serologic response to mRNA COVID‐19 vaccination in lymphoma patients. Am J Hematol. 2021;96(11):E410-3.
Lim SH, Campbell N, Johnson M, et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8(8):e542-4.
Mairhofer M, Kausche L, Kaltenbrunner S, et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell. 2021;39(9):1171-2.
Malard F, Gaugler B, Gozlan J, et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021;11(8):142.
Marasco V, Carniti C, Guidetti A, et al. T‐cell immune response after mRNA SARS‐CoV‐2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol. 2022;196(3):548-58.
Marchesi F, Pimpinelli F, Sperandio E, et al. The 12‐week kinetics of anti‐SARS‐CoV‐2 antibodies in different haematological cancers after vaccination with BNT162b2. Br J Haematol. 2022;196(2):362-7.
McKenzie DR, Muñoz-Ruiz M, Monin L, et al. Humoral and cellular immunity to delayed second dose of SARS-CoV-2 BNT162b2 mRNA vaccination in patients with cancer. Cancer Cell. 2021;39(11):1445-7.
Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765-78.
Parry H, McIlroy G, Bruton R, et al. Impaired neutralisation of SARS-CoV-2 delta variant in vaccinated patients with B cell chronic lymphocytic leukaemia. J Hematol Oncol. . 2022;15(1):1-2.
Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11(7):136.
Peeters M, Verbruggen L, Teuwen L, et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6(5):100274.
Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053-61.
Pimpinelli F, Marchesi F, Piaggio G, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14(1):1-2.
Rahav G, Lustig Y, Lavee J, et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: a prospective cohort study. EClinicalMedicine. 2021;41:101158.
Schiller Salton NS, Szwarcwort M, Tzoran I, et al. Attenuated humoral immune response following anti‐SARS‐CoV‐2 vaccine in heavily pretreated patients with multiple myeloma and AL amyloidosis. Am J Hematol. 2021;96(12):E475-8.
Shem‐Tov N, Yerushalmi R, Danylesko I, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in haematopoietic stem cell transplantation recipients. Br J Haematol. 2022;196(4):884-91.
Shen Y, Freeman JA, Holland J, et al. COVID‐19 vaccine failure in chronic lymphocytic leukaemia and monoclonal B‐lymphocytosis; humoural and cellular immunity. Br J Haematol. 2022;197(1):41-51.
Stampfer SD, Goldwater MS, Jew S, et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia. 2021;35(12):3534-41.
Terpos E, Gavriatopoulou M, Fotiou D, et al. Poor neutralizing antibody responses in 132 patients with CLL, NHL and HL after vaccination against SARS-CoV-2: a prospective study. Cancers. 2021;13(17):4480.
Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11(8):138.
Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674-6.
Thakkar A, Gonzalez-Lugo JD, Goradia N, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081-90.e2.
Van Oekelen O, Gleason CR, Agte S, et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028-30.
Greenberg RS, Ruddy JA, Boyarsky BJ, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with multiple myeloma. BMC Cancer. 2021;21(1):1354.
Arruga F, Gyau BB, Iannello A, et al. Immune response dysfunction in chronic lymphocytic leukemia: dissecting molecular mechanisms and microenvironmental conditions. Int J Mol Sci. 2020;21(5):1825.
Noori M, Azizi S, Abbasi Varaki FA, et al. A systematic review and meta-analysis of immune response against first and second doses of SARS-CoV-2 vaccines in adult patients with hematological malignancies. Int Immunopharmacol. 2022;110:109046.
Dhakal B, Abedin S, Fenske T, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138(14):1278-81.
Marra AR, Kobayashi T, Suzuki H, et al. Short-term effectiveness of COVID-19 vaccines in immunocompromised patients: A systematic literature review and meta-analysis. J Infect. 2022;84(3):297-310.