Corresponding author: Kosuke Obama, patagonta2008@yahoo.co.jp
DOI: 10.31662/jmaj.2024-0028
Received: February 19, 2024
Accepted: March 19, 2024
Advance Publication: June 3, 2024
Published: July 16, 2024
Cite this article as:
Obama K, Yamamoto H, Inoue H. Lenalidomide-induced Pseudogout and Crowned Dens Syndrome. JMA J. 2024;7(3):445-446.
Key words: lenalidomide, pseudogout, crowned dens syndrome, myeloma
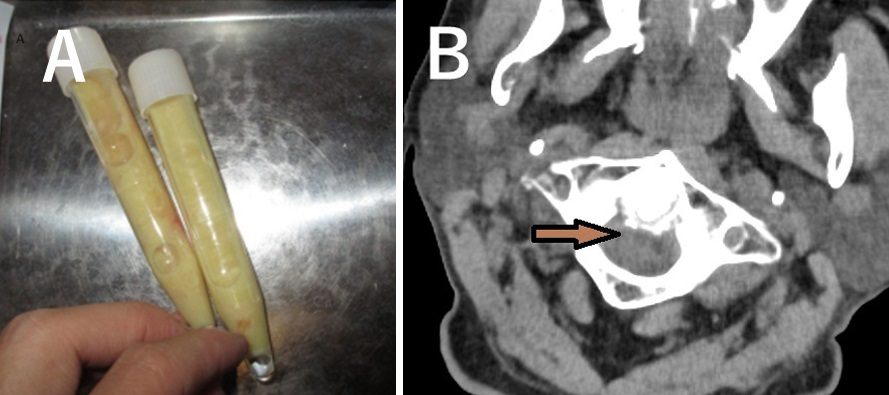
An 84-year-old male diagnosed with IgA myeloma was treated with lenalidomide. The disease was managed adequately; however, after 11 months, he experienced high-grade fever, swelling and pain in his right elbow, and severe posterior neck pain. A cloudy joint fluid was identified (Figure 1A), and the patient was diagnosed with pseudogout. Moreover, he was diagnosed with crowned dens syndrome based on cervical spine findings of computed tomography (Figure 1B). Discontinuation of lenalidomide and administration of nonsteroidal anti-inflammatory drugs reduced these symptoms. Nine months after the lenalidomide cessation, myeloma worsened, and readministration of lenalidomide led to generalized arthralgia and severe swelling of the left ankle joint; lenalidomide-induced arthritis occurrence was clinically confirmed. In Japan, 24, 8, and 1 cases of mild arthralgia, arthritis, and joint swelling, respectively, have been reported. Notably, some joint symptoms can be associated with lenalidomide-associated pseudogout.
None
Kosuke Obama: planning and conducting research and writing the manuscript
Hana Yamamoto and Hirosaka Inoue: treatment coresponsibility
Kosuke Obama: 0000-0001-6717-1531
Approval from the ethical board was not required.
Written informed consent was obtained from the patient by the corresponding author.