Corresponding author: Katsuhisa Yamada, yka2q@pop.med.hokudai.ac.jp
DOI: 10.31662/jmaj.2024-0048
Received: March 11, 2024
Accepted: March 12, 2024
Advance Publication: June 10, 2024
Published: July 16, 2024
Cite this article as:
Yamada K, Sudo H, Iwasaki N. Reverse Translational Approach Using Biomaterials and Stem Cells for Intervertebral Disc Degeneration. JMA J. 2024;7(3):423-425.
Key words: intervertebral disc degeneration, low back pain, soft biomaterial, mesenchymal stem cell
Low back pain is a major health problem that interferes with daily life. Intervertebral disc (IVD) degeneration is a major cause of low back pain. Although analgesics and physical therapy are commonly used to manage chronic low back pain associated with IVD degeneration, neither of these approaches directly addresses the etiology of symptoms (1), (2). Surgical treatment for spinal disorders can also provide benefits. However, a treatment that can control IVD degeneration itself has not yet been developed, and these limitations have drawn attention to the potential of alternative therapies (1), (2). Recently developed tissue engineering approaches have revealed the molecular cascade involved in the degeneration of IVDs, and regenerative therapies using cells and biomaterials have gained attention as treatments aimed at regenerating degenerated IVDs (1), (2), (3).
The IVD consists of nucleus pulposus (NP) and annulus fibrosus. NP is a gelatinous tissue that contains 70%-90% of water; it is rich in extracellular matrix but low in cell density (1). IVD degeneration is characterized by loss of water content and extracellular matrix degradation, and it is considered that degenerated IVDs do not spontaneously regenerate due to poor nutrient supply and low cell mitotic capacity (4), (5). Thus, the development of therapies aimed at regenerating NP could help improve the function of IVDs (5).
As a development approach to the next-generation treatment for IVD disorders, the possibility of regulating IVD degeneration from the biomechanical and molecular biological aspects of IVD degeneration has been demonstrated (Figure 1, left) (1). Because IVD cell apoptosis is a characteristic phenomenon that occurs in the early stages of IVD degeneration (1), (6), it has been demonstrated that genetically controlling IVD cell apoptosis may be beneficial in IVD tissue degeneration (4), (5), (6), (7). As a biomaterial for tissue repair, the administration of a highly purified alginate-based hardening gel (UPAL) alone (cell-free) after discectomy was found to optimize the tissue repair environment in vivo and promote the self-repair ability of the residual tissue, resulting in the spontaneous repair of the IVD (Figure 1, left) (8). An in vivo study of rabbits implanted with UPAL hydrogel after IVD aspiration exhibited a significant increase in the percentage of GD2+Tie2+ cells (8), which are NP progenitor cells (9), suggesting that the implanted soft biomaterial induces endogenous NP cells and NP progenitor cells, leading to endogenous IVD repair (1), (8). Based on these results, a clinical trial was conducted to implant UPAL gel into the disc after discectomy in patients undergoing surgery for lumbar disc herniation (10). In addition, focusing on the “pain” associated with IVD, the UPAL gel suppressed the expression of inflammatory cytokines and nerve growth factor receptors, demonstrating its usefulness in reducing discogenic back pain (Figure 1, right) (11).
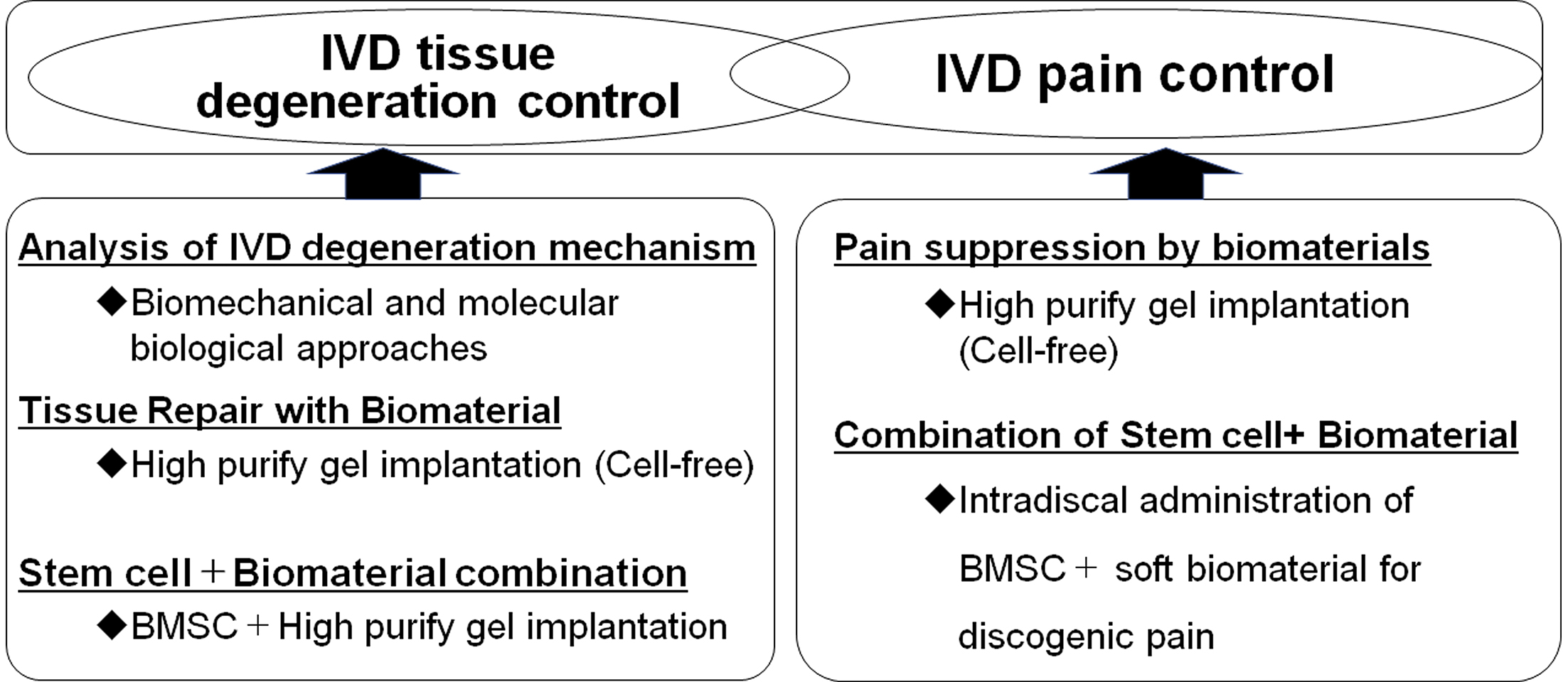
However, there are clear limitations to treatment with biomaterials alone in middle-aged and older patients with poor self-repair capacity. Thus, we focused on stem cells as a treatment for IVD regeneration (1), (3), of which we investigated the use of bone marrow-derived mesenchymal stem cells (BMSCs) (12). The efficacy of intradiscal administration of BMSCs in patients with chronic low back pain associated with disc degeneration has been reported, and several clinical trials have been conducted (1), (2). Implantation of a mixture of allogeneic BMSCs and UPAL gel in degenerated IVDs was found to promote tissue repair compared with gel alone in in vivo studies (Figure 1, left) (12). Regarding the mechanism of IVD regeneration by BMSCs and biomaterials, the expression of NP cell markers increased over time in transplanted BMSCs in these studies, indicating the differentiation of transplanted BMSCs into NP cells in vivo (12). Furthermore, high type II collagen-positive cell rates were observed in IVDs transplanted with BMSCs in combination with gel, indicating the enhanced production of extracellular matrix in discs transplanted with allogeneic BMSCs (12). However, the problems with conventional BMSCs include cell quality degradation during cell culture, difficulty in maintaining cell quality, and the presence of nondifferentiating contaminating cells (2), (13). Rapidly expanding clones (RECs) are high-quality, highly purified, allogeneic human BMSC cells isolated and extracted directly from the bone marrow using a cell sorter based on the expression of two cell surface markers, namely, CD271 and CD90 (13), (14). RECs are genetically stable, ultrapurified, Good Manufacturing Practices-compliant clonogenic BMSCs and do not show lot-related differences in clinical applications (13), (14). Implantation of REC in combination with UPAL gel into the IVD was found to promote tissue repair in a sheep model of severe IVD degeneration compared with gel alone, indicating that UPAL gel prevents cell leakage as a cell carrier and facilitates BMSC activation (1), (13). A prospective, double-blind, randomized, controlled trial of REC in combination with UPAL gel has also been conducted in middle-aged and older patients with lumbar spinal canal stenosis complicated by disc herniation (15).
Basic research was conducted from the perspective of a reverse translational approach to elucidate the effects of the combination of biomaterials and BMSCs on pain suppression (Figure 1, right). To develop a novel local injection therapy for discogenic low back pain, the biomaterial was designed to be injected into the IVD in a sol rather than a gel state. Injection of a mixture of REC and UPAL nongelling solution in a rat caudal IVD punch model suppressed IVD degeneration as well as the expressions of inflammatory cytokines and nerve growth factor receptors (TNF-α, IL-6, and TrkA) (2). Moreover, a mixture of REC and UPAL suppressed nociceptive behavior compared with UPAL alone (2). This research has the great advantage of being clinically applicable as it used stem cells and soft biomaterials that have been clinically used in humans. The results indicate that the method of a single injection of stem cells and soft biomaterial has a potential application in the treatment of discogenic pain and as a regenerative therapy (2). This method is minimally invasive and safe, does not require hospitalization or surgery, and could offer greater pain control and disc regeneration than existing treatments for low back pain (2).
Thus, from the perspective of the reverse translational approach, which aims to return to basic research and elucidate the mechanisms of the issues raised by the results of clinical studies, the development of a new next-generation treatment that suppresses IVD tissue degeneration and is useful in reducing discogenic low back pain is expected.
This article is based on the study, which received the Medical Research Encouragement Prize of The Japan Medical Association in 2023.
None
Katsuhisa Yamada: Conceptualization, Methodology, Formal analysis, Writing - original draft, Investigation; Hideki Sudo: Conceptualization, Methodology, Formal analysis, Writing - review and editing, Supervision, Project administration; Norimasa Iwasaki: Supervision.
Yamada K, Iwasaki N, Sudo H. Biomaterials and cell-based regenerative therapies for intervertebral disc degeneration with a focus on biological and biomechanical functional repair: targeting treatments for disc herniation. Cells. 2022;11(4):602.
Suzuki H, Ura K, Ukeba D, et al. Injection of ultra-purified stem cells with sodium alginate reduces discogenic pain in a rat model. Cells. 2023;12(3):505.
Ukeba D, Yamada K, Tsujimoto T, et al. Bone marrow aspirate concentrate combined with in situ forming bioresorbable gel enhances intervertebral disc regeneration in rabbits. J Bone Joint Surg Am. 2021;103(8):e31.
Sudo H, Minami A. Regulation of apoptosis in nucleus pulposus cells by optimized exogenous Bcl-2 overexpression. J Orthop Res. 2010;28(12):1608-13.
Sudo H, Minami A. Caspase 3 as a therapeutic target for regulation of intervertebral disc degeneration in rabbits. Arthritis Rheum. 2011;63(6):1648-57.
Yamada K, Sudo H, Iwasaki K, et al. Caspase 3 silencing inhibits biomechanical overload-induced intervertebral disk degeneration. Am J Pathol. 2014;184(3):753-64.
Ohnishi T, Yamada K, Iwasaki K, et al. Caspase-3 knockout inhibits intervertebral disc degeneration related to injury but accelerates degeneration related to aging. Sci Rep. 2019;9(1):19324.
Tsujimoto T, Sudo H, Todoh M, et al. An acellular bioresorbable ultra-purified alginate gel promotes intervertebral disc repair: a preclinical proof-of-concept study. EBioMedicine. 2018;37:521-34.
Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264.
Yamada K, Kenichiro M, Ito YM, et al. Exploratory clinical trial on the safety and capability of dMD-001 in lumbar disc herniation: study protocol for a first-in-human pilot study. Contemp Clin Trials Commun. 2021;23:100805.
Ura K, Yamada K, Tsujimoto T, et al. Ultra-purified alginate gel implantation decreases inflammatory cytokine levels, prevents intervertebral disc degeneration, and reduces acute pain after discectomy. Sci Rep. 2021;11(1):638.
Ukeba D, Sudo H, Tsujimoto T, et al. Bone marrow mesenchymal stem cells combined with ultra-purified alginate gel as a regenerative therapeutic strategy after discectomy for degenerated intervertebral discs. EBioMedicine. 2020;53:102698.
Ukeba D, Yamada K, Suyama T, et al. Combination of ultra-purified stem cells with an in situ-forming bioresorbable gel enhances intervertebral disc regeneration. EBioMedicine. 2022;76:103845.
Mabuchi Y, Morikawa S, Harada S, et al. LNGFR(+)THY-1(+)VCAM-1(hi+) cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Rep. 2013;1(2):152-65.
Sudo H, Miyakoshi T, Watanabe Y, et al. Protocol for treating lumbar spinal canal stenosis with a combination of ultrapurified, allogenic bone marrow-derived mesenchymal stem cells and in situ-forming gel: a multicentre, prospective, double-blind randomised controlled trial. BMJ Open. 2023;13(2):e065476.