Corresponding author: Taku Sugiyama, takus1113@med.hokudai.ac.jp
DOI: 10.31662/jmaj.2024-0141
Received: June 28, 2024
Accepted: July 17, 2024
Advance Publication: August 30, 2024
Published: January 15, 2025
Cite this article as:
Sugiyama T, Sugimori H, Tang M, Fujimura M. Artificial Intelligence for Patient Safety and Surgical Education in Neurosurgery. JMA J. 2025;8(1):76-85.
Neurosurgery has evolved alongside technological innovations; however, these advances have also introduced greater complexity into clinical practice. Neurosurgery remains a demanding and high-risk field that requires a broad range of skills. Artificial intelligence (AI) has immense potential in neurosurgery given its ability to rapidly analyze large volumes of clinical data generated in modern clinical environments. An expanding body of literature has demonstrated that AI enhances various aspects of neurosurgery, including diagnostics, prognostication, decision-making, data management, education, and clinical studies. AI applications are expected to reduce medical errors and costs, broaden healthcare accessibility, and ultimately boost patient safety and surgical education. Nevertheless, AI application in neurosurgery remains practically limited because of several challenges, such as the diversity and volume of clinical training data collection, concerns regarding data quality, algorithmic bias, transparency (explainability and interpretability), ethical issues, and regulatory implications. To comprehensively discuss the potential benefits, future directions, and limitations of AI in neurosurgery, this review examined recent studies on AI technology and its applications in this field, focusing on intraoperative decision support and surgical education.
Key words: AI, computer vision, deep learning, machine learning, microsurgery, surgical skill
Advances in neurosurgery have closely paralleled technological developments, which have significantly improved patient outcomes in contemporary neurosurgery practices (1). However, these advancements have increased complexity by incorporating multiple technologies and fostering multidisciplinary approaches (2), (3).
In modern operating rooms, neurosurgeons are equipped with an array of surgical devices and monitors and collaborate with diverse teams, including anesthesiologists, technicians, and nurses. The role of neurosurgeons requires not only a profound understanding of various neurological disorders, including demographics, etiology, genetics, pathophysiology, diagnostic techniques, and prognosis, but also expertise in complex neuroanatomy, diverse surgical procedures, advanced psychomotor skills, and leadership in communication with other staff members. Neurosurgery remains a demanding field that requires mastery of cognitive, decision-making, and technical surgical skills through extensive, intensive, and prolonged training (4). Neurosurgeons must assimilate, retain, analyze, and interpret vast, dynamic, and complex datasets and tasks that increasingly challenge human capabilities. Moreover, when treating patients in the acute phase, quick decision-making is required in many clinical settings. Reducing door-to-intervention time during emergency procedures often compromises the time available for physicians to process enormous amounts of clinical data.
Given the aforementioned challenges and critical significance of the central nervous system, the incidence of adverse events in neurosurgical procedures might be notably higher than that in other medical disciplines, significantly affecting patients’ quality of life (5), (6), (7). The literature indicates that approximately 25% of these events are attributable to preventable technical errors, underscoring the need for systematic measures to mitigate such medical errors and ensure patient care that yields satisfactory outcomes.
Among the most expected recent advancements in neurosurgery, artificial intelligence (AI) applications include machine learning (ML), natural language processing (NLP), computer vision (CV), and artificial neural networks (ANNs) (8). The potential of AI in neurosurgery is substantial and is supported by its ability to rapidly analyze large volumes of clinical data generated in contemporary settings, which surpass human capabilities. Specialized algorithms can be applied to diverse multimodal clinical data sources, including alphanumeric electronic health charts, laboratory testing, radiological images, electrophysiological examinations, and clinical data from photographs and videos. A previous study indicated that AI can improve various aspects of neurosurgery, including diagnostics, prognostication, clinical decision-making, data management, education, and clinical research (8). AI applications can reduce medical errors, decrease costs, broaden healthcare availability, and enhance patient safety and surgical education.
However, the practical application of AI in neurosurgery remains limited because of several challenges, such as the diversity and volume of clinical training data collection, concerns regarding data quality, algorithmic bias, transparency (explainability and interpretability), ethical issues, and regulatory implications. This review comprehensively examines recent studies on AI technology and its applications to better understand its potential benefits, future perspectives, and limitations in neurosurgery.
As exemplified by the computer-assisted diagnosis of gliomas (9), AI has been frequently used to aid diagnostic approaches. The capability of AI to rapidly process enormous amounts of anatomical, morphological, and connectivity information from imaging data makes computer-assisted diagnosis one of the most promising areas for AI applications, assisting neuroradiologists and neurosurgeons, and reducing human labor and costs. Gliomas have been successfully detected and differentiated from other central nervous system pathologies (10). Tumor grading and prediction of isocitrate dehydrogenase mutation (11), 1p19q co-deletion (12), O-6-methylguanine-DNA-methyltransferase promoter status (13), and overall survival rates have been achieved using predictive AI models (9), (14). Combining radiomics data with clinical variables has facilitated the development of various ML models that can reliably differentiate the molecular subgroups of brain tumors (15).
Similarly, for the detection of intracranial aneurysms, deep learning (DL) algorithms have been found to be more efficient than radiologists (16). The prediction of aneurysm stability has also been investigated using prediction models (17), (18). Applications of AI in the fields of functional and spinal surgeries have led to achievements in electroencephalogram analysis, seizure categorization, epilepsy subtype classification, automated detection of seizures in videos, and detection of lesions compressing the spinal cord (19), (20), (21).
AI applications in aiding the diagnosis of radiological imaging have been well documented in the literature and have reached the stage of broad clinical application. However, to effectively train, validate, and test diagnostic AI systems, well-annotated datasets that include imaging data and tissue samples are required. These datasets are costly and time-consuming. Additionally, many intracranial diseases, including glioma, are relatively rare, making the acquisition of large volumes of clinical data a significant challenge (9).
Predicting outcomes, postoperative complications, and adverse events remains a critical focus for neurosurgeons. A previous study revealed that the gradient boosting ML algorithm outperformed traditional statistical methods in predicting early postoperative complications following intracranial tumor surgery (22). Another study successfully used conditional ML algorithms and inference tree analysis to predict perioperative transfusion needs among adult patients undergoing spinal deformity surgery (23). ML technologies have been extensively tested for various neurosurgical scenarios, including predicting survival rates following brain metastasis resection (24), seizure-free outcomes after anterior temporal lobectomy (25), short-term mortality and adverse events after spinal surgery (26), the likelihood of aneurysm rupture, the outcomes and incidence of delayed cerebral ischemia following subarachnoid hemorrhage (27), (28), and clinical outcomes following flow diversion stent treatments for intracranial aneurysms (29).
Although these results are promising, a recent systematic review revealed that the majority of these studies were single-center investigations, with only 15% of them conducting external validation using an independent cohort of patients (30). This lack of transparency in the underlying logic of ML models makes most clinicians hesitant to trust their predictions provided by ML models (31). Similar to traditional statistical methods, such as logistic regression, explainability, and interpretability, future AI models should address these challenges. Another inherent limitation of these studies was that most risk predictions were provided preoperatively. However, most risky intraoperative events occur unexpectedly, and in practical clinical situations, the surgical team must manage these events reactively by relying on clinical assessment. Therefore, real-time feedback in the operating room is critical for clinical decision support using AI is required.
The primary objective of neurosurgery is maximal resection or eradication of pathological lesions while preserving neurological function. AI has the potential to enhance surgical performance and reduce the risk of medical errors during the intraoperative phase of neurosurgery. Shen et al. and Hollon et al. implemented deep convolutional neural networks in conjunction with indocyanine green fluorescence imaging and Raman spectroscopy to facilitate near-real-time intraoperative glioma diagnosis (32), (33). These methodologies enable tumor diagnosis within 3 min, which is a significant improvement over the traditional rapid frozen section pathological diagnosis, which typically requires up to 30 min. Similarly, Cakmakci et al. used a random forest model in conjunction with high-resolution magic angle spinning nuclear magnetic resonance spectroscopy to accurately determine the metabolic profile of the resected tissue intraoperatively, identify residual tumorous tissue in the excision cavity, and guide surgeons toward maximal resection (34). Qiao et al. used automated DL analysis for intraoperative visually evoked potentials during endoscopic transsphenoidal surgery, thereby preventing optic nerve injury and reducing labor-intensive work by electrophysiologists (35).
The utility of AI extends to surgical planning, particularly in determining the appropriate surgical approach, and it holds promise for improving outcomes and minimizing neurological sequelae. Dundar et al. proposed a surgical route planning algorithm that combines heuristics with Q-learning, a reinforcement learning-based AI technique, to identify optimal skull entry points and routes for minimally invasive tumor removal (36). Given that intraoperative brain shifts pose a significant challenge during cranial surgery, Tonutti et al. used ANNs and support vector regression in combination with the finite element method (FEM) to predict the deformation at each node in a brain tumor mesh model. They reported on-screen positional errors of <0.4-0.5 and 0.2 mm compared with an average error of 1.4 mm for other deformation models (37). These results highlight that ML algorithms can be applied to enhance FEM-based biomechanical simulations of anatomical structures, allowing for real-time responses (38).
Automated operative workflow analysis using CV technologies has also been applied to surgical procedures, such as endoscopic transsphenoidal surgery. The ML model was able to distinguish the various steps and techniques for excision of pituitary adenoma with high accuracy, even with significant variability in the training data (39). Disruptions in procedural flow during surgery can lead to errors and increase the risk of complications. Common causes include teamwork or communication failures, technology and equipment malfunctions, and training-related distractions.
Currently, most surgical procedures are condensed into a single-page operative report that serves as the standard information source for all surgical disciplines. Despite the availability of surgical video recordings and data from multiple monitoring systems. However, a previous study showed that these surgical reports failed to detail nearly one-third of intraoperative adverse events. The current paradigm of “surgical data science” emphasizes the importance of evaluating both how the operation proceeds and how effectively the surgeon performed the surgery (40), (41). Given the massive amount of data, including monitoring data and video recordings, the incorporation of AI technologies into data acquisition, curation, and analysis is considered the most promising solution to this problem. The major obstacles to the widespread use of AI are as follows: (1) anatomical structures in the surgical field are often hidden under layers of other tissues or obscured by a surgeon’s hands, making it difficult to train models for detecting specific anatomical structures or surgical instruments; and (2) there is significant variation, even among expert surgeons, in how they perform surgical procedures, necessitating comprehensive datasets that encompass various surgical expertise.
Surgeon proficiency plays a pivotal role in the outcomes of surgical procedures and significantly influences adverse events, morbidity, and mortality (42). Implementing comprehensive evaluations of surgical skills provides a reliable measure that not only identifies and corrects deficiencies in surgical trainees through constructive feedback but also accredits surgeon competence in society, thereby enhancing both surgical education and patient safety (43).
Traditional assessment of surgical skills relied primarily on subjective evaluations by the surgeon. Several criteria-based scoring systems were also used in these evaluations. However, these methods are limited by the significant human and time resources required and the potential for inter-rater bias (43). Efforts have been made to develop quantifiable evaluation methodologies that address subjectivity. Notably, engineering technologies using specialized tools, such as sensors affixed to surgical instruments to measure force (43), (44) or electromagnetic trackers to monitor the movements of surgeons’ hands or instruments (45), have been developed. Recently, AI technologies have been used for data analysis to quickly provide feedback with minimal human resources (46), (47). Another innovative approach involves the use of virtual reality (VR) simulators (48), (49). These simulators are particularly effective for repeated training sessions and facilitate easy data collection, although they may not fully capture the physical characteristics of tissues and psychological pressures present in real surgical situations (50).
Although these methods facilitate an objective assessment of skill levels, their reliance on specialized equipment, such as sensors or VR simulators, limits their widespread adoption and raises concerns about their generalizability and reproducibility. In recent years, there has been a shift toward analytical methods that leverage video recordings of surgical procedures. The proposed method is gaining traction because of its applicability to a broad array of operating room environments (51). Additionally, the use of AI technologies to evaluate surgical skills via video analysis is becoming increasingly common (40), (52).
Pangal et al. used an automated instrument detection algorithm to calculate performance metrics for predicting overall success rates, blood loss, and hemorrhage control within <1 min of endoscopic endonasal surgery using a cadaveric internal carotid artery vascular injury simulator (53). The findings indicate that these performance metrics are more reliable predictors of surgical outcomes than the training status or prior experience of the surgeons (53). Our group also developed an automated instrument detection algorithm for carotid endarterectomy (Figure 1) (52). Additionally, we proposed a novel metric for the objective and quantitative assessment of surgical skills by focusing on tissue motion during surgical maneuvers (Figure 2). Analysis of the motion parameters (e.g., acceleration and velocity) of target tissues (carotid plaques) during carotid endarterectomy revealed that novice surgeons typically exhibit higher tissue motion parameter values than expert surgeons (54), (55). Additionally, this metric correlates with adverse events (ischemic stroke and cervical nerve injury) during surgery (54). These findings indicate that minimizing tissue movement during surgery can serve as a novel indicator of surgical skill and outcomes, emphasizing “gentleness” in tissue handling (54), (55).
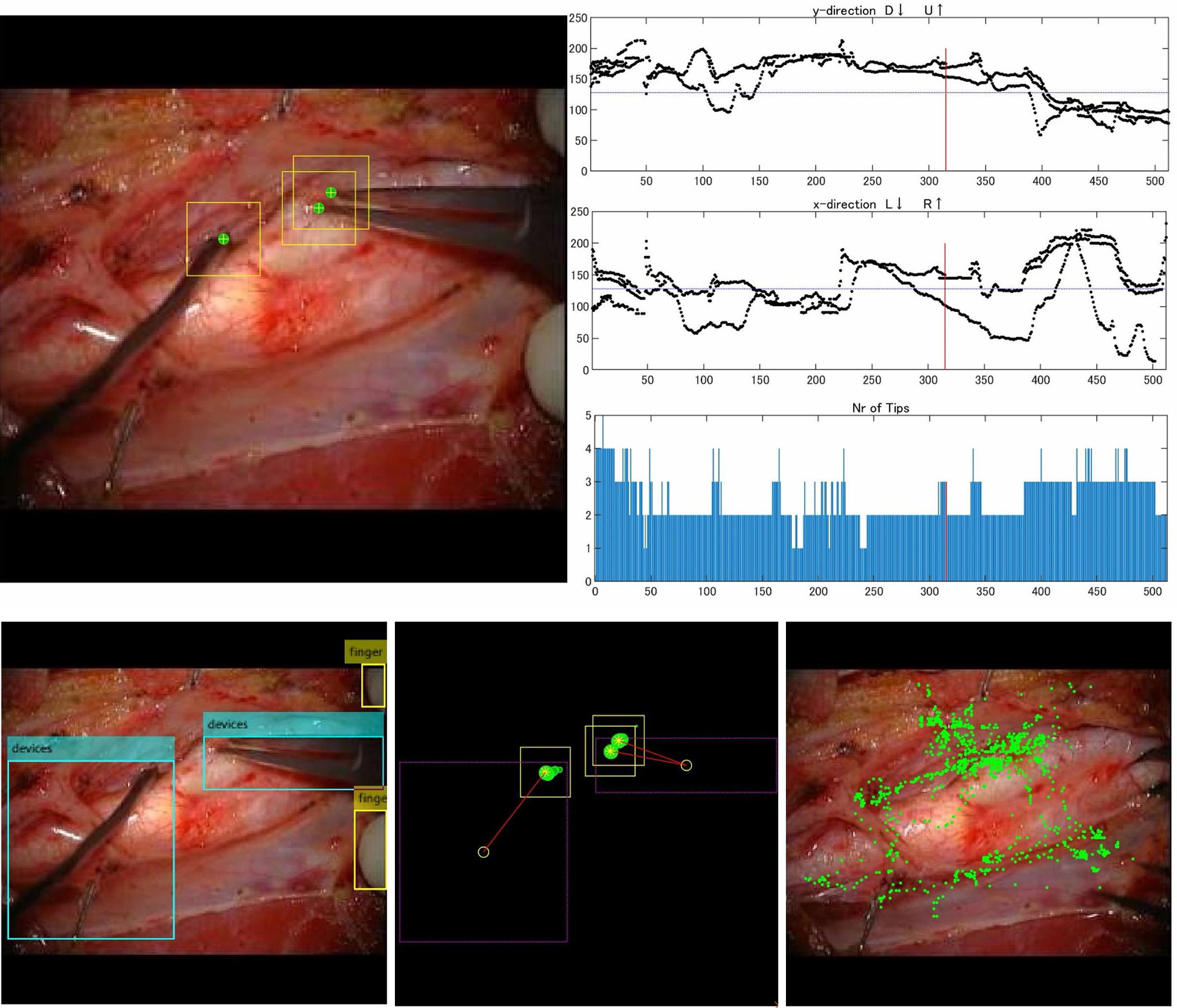
As microvascular procedures require specialized technical skills to handle fragile intracranial arteries, several AI applications have been reported for microvascular anastomosis training. Gonzalez-Romo et al. developed an ML-based model capable of tracking 21 hand landmarks for microvascular anastomosis simulation (56). Recently, our study group developed and used an automated instrument tip-tracking algorithm to measure motion economy (procedural time and path distance) and motion smoothness (normalized jerk index) during the task of suturing artificial blood vessels (Figure 3) (57). Similar findings have been observed in microsurgical arachnoid dissection (58).
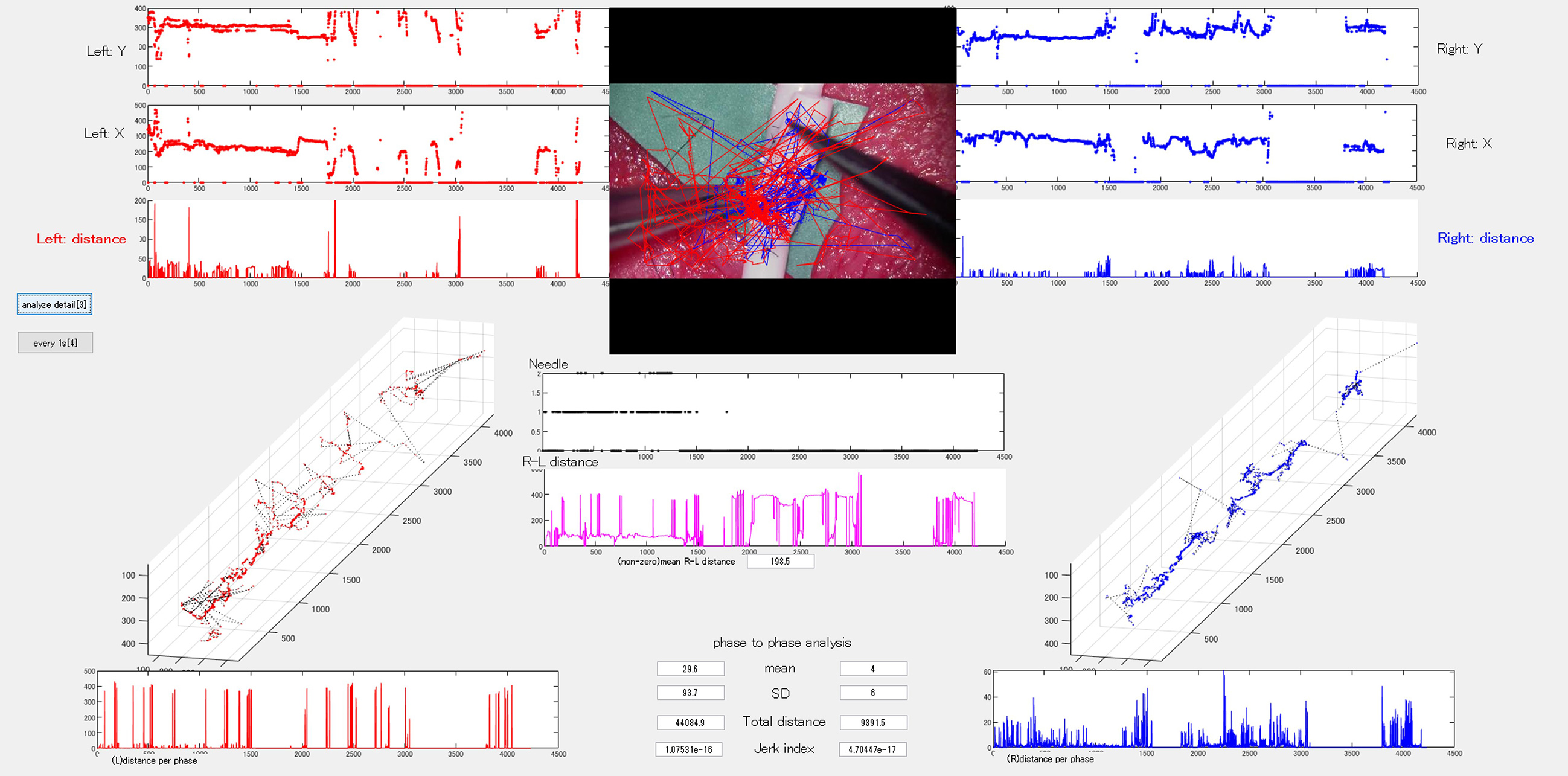
Additionally, we developed a DL-based semantic segmentation algorithm to assess changes in vessel area under the hypothesis that avoiding excessive or abrupt vessel deformation could serve as a novel metric for gentle maneuvering during microvascular anastomosis (Figure 4) (59). By applying these models, we demonstrated differences between experts and novices in terms of video parameters and provided technical challenges for trainee surgeons. These studies did not evaluate training efficiency through repeated training sessions after providing feedback on their techniques; thus, further studies are necessary to validate training efficiency and estimate learning curves.
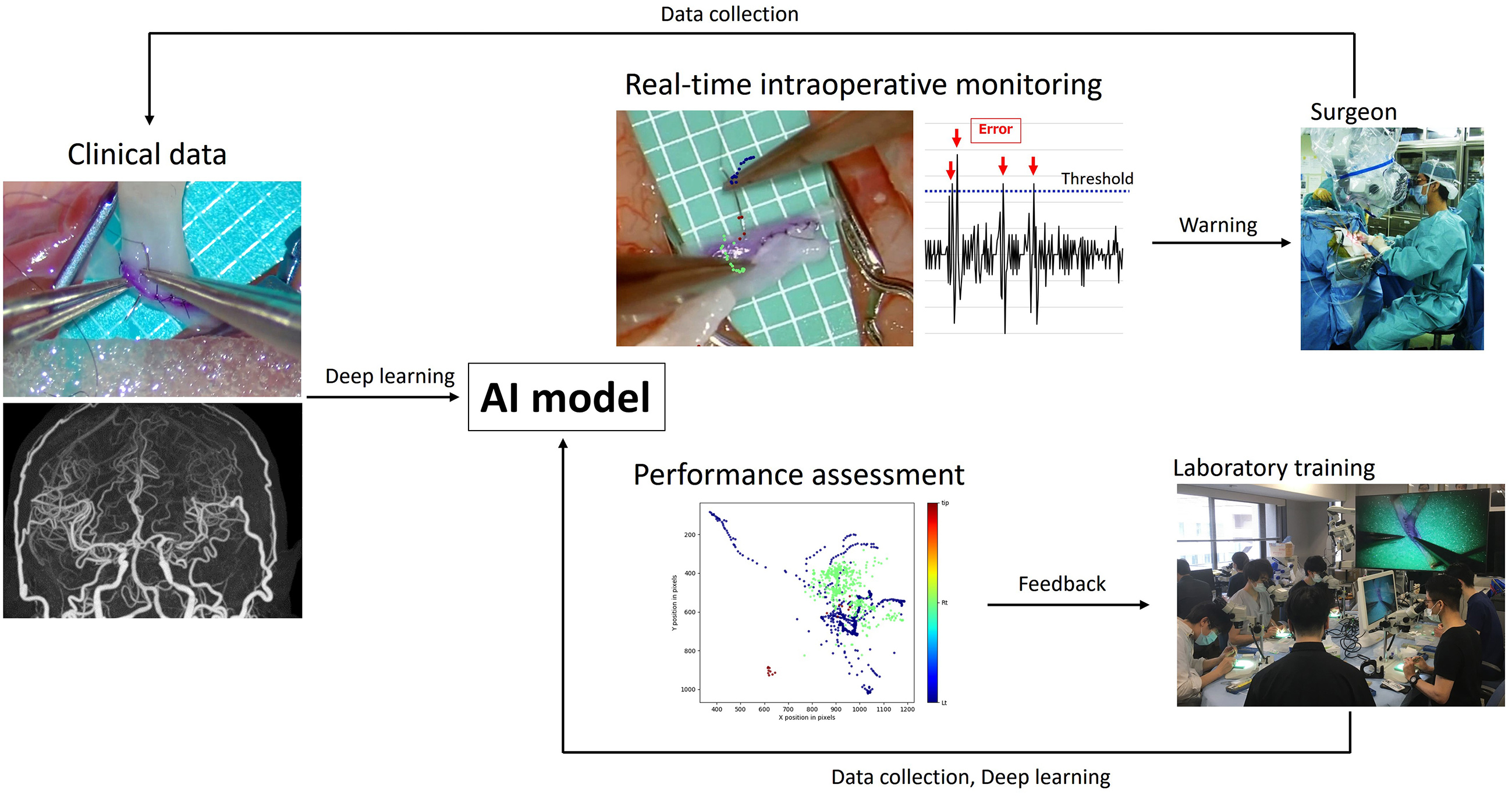
A data-driven analysis of surgical performance can numerically define several surgical errors that have been proven to be correlated with intraoperative adverse events (44), (54), (59). Therefore, AI-powered real-time monitoring with warning systems should be developed and introduced in clinical practice (Figure 5). However, given the extensive variety of technical surgical nuances, further exploratory data-driven studies should be conducted to identify metrics that align surgical proficiency with patient outcomes.
Large language models (LLMs) are generative AI models that produce human-like texts. Recently, their popularity has surged, with significant applications in studies, education, and clinical tasks (60). This trend extends to neurosurgery, in which LLMs are actively integrated into daily practice.
Previous investigations into the capabilities of ChatGPT (versions GPT-3.5 and GPT-4.0) and Google Bard have shown high performance in U.S. medical licensing examination- and neurosurgical board examination-style questions, demonstrating their potential utility in standardized medical testing (61), (62), (63). In neurosurgical studies and documentation, ChatGPT has been used to facilitate the accurate coding of neurosurgical procedures, highlighting its broad logistical utility (64).
In clinical applications, LLMs have shown promising diagnostic capabilities. For example, GPT-4.0 demonstrated an accuracy of up to 62% in classifying specific types of tumors during neuroradiological evaluation (65). Furthermore, LLMs have been used in treatment queries (66), (67), neurosurgical triage and consultation (68), and procedural inquiries (69), indicating their potential use as clinical and diagnostic aids. The development of neuroGPT-X has demonstrated performance on par with or superior to that of expert responses in terms of coherence, thoroughness, relevance, and accuracy (70). These applications highlight the need for this technology and its capacity to deliver prompt and readily available clinical information to patients, students, and healthcare professionals. LLMs can promote the synthesis of academic studies and their translation into clinical practice (10).
Additionally, NLP techniques are used to analyze free text in patient medical records. NLP, a subfield of AI, uses tools to encode concepts and derive meanings from unstructured text, thereby enhancing the understanding of patient data (71). Tirukumaran et al. discovered that models using NLP could predict surgical site infections in orthopedic surgery patients with a level of accuracy similar to that of manual data extraction and better than models relying solely on administrative data (72).
The integration of AI into neurosurgery presents significant challenges and risks that must be addressed to optimize outcomes and maintain ethical standards. One major challenge is to ensure that both patients and healthcare providers can effectively interpret and understand AI-driven models and the implications for treatment and predictive outcomes. Despite the high accuracy of their predictions, AI systems are not without errors or uncertainties, and uncritical reliance on these technologies can lead to undue dependency and potential mismanagement (8).
The complex nature of neural networks, with their multiple processing layers, often remains opaque, creating what is commonly referred to as an “algorithmic black box.” Understanding these mechanisms is crucial when using AI in clinical settings. Additionally, AI systems generally lack the versatility of human analytical skills because many clinical AI applications are designed to perform specific tasks within limited contexts. Training algorithms for data from specific institutions or patient demographics can introduce inherent biases that reflect the characteristics of the training data rather than a general patient population. Explainable AI is a promising solution to these issues (73), (74). However, the limited applicability of current AI models highlights the need for enhanced human oversight to prevent errors.
Furthermore, excessive reliance on AI can diminish the clinical expertise and decision-making capabilities of surgeons. Dependence on AI recommendations without a thorough evaluation can result in overlooked diagnoses or ineffective treatment strategies (8), (20).
Another critical concern is cybersecurity (20). AI systems are networked; thus, vulnerabilities may lead to unauthorized access, data manipulation, or disruptions during surgical procedures. The necessity for vast amounts of patient data to develop robust AI models also raises ethical concerns regarding data privacy.
A significant array of clinically relevant algorithms must be implemented. The debate over whether patient data should be recorded is ongoing; however, it is crucial to address the ethical and legal implications of potential AI-driven misdiagnoses. Moreover, clinicians require computer science training to better understand and leverage these AI systems.
AI has already demonstrated diagnostic and prognostic accuracy comparable with that of experienced clinicians. A promising approach for enhancing clinical diagnosis is to integrate the expertise of experienced clinicians into AI algorithms. By incorporating insights from these experts, neurosurgical trainees can gain valuable guidance to develop their clinical skills.
In neurosurgery, AI can also enhance the function of surgical adjuncts. For example, semiautomated positioning of endoscopes or microscopes using predefined anatomical landmarks derived from imaging has shown potential for intracranial and spinal surgeries (4). Additionally, AI can improve image-guidance systems using preoperative or intraoperative imaging data to assist in planning surgical paths. Automated systems can further aid surgeons by providing alerts when critical structures, such as blood vessels and sensitive areas, are at risk. AI-powered neurosurgical VR simulators can efficiently and accurately assess the skills of residents and medical students.
Surgical robots that offer mechanical advantages over humans can be further improved. By combining robotic assistance with advanced sensory technologies, such as machine vision, haptic feedback, motion sensors, and automated control algorithms, human surgical capabilities can be extended beyond their current limits (1), (8). Furthermore, integrating brain-computer interfaces and AI can restore sensory and motor functions in patients with paralysis, enhance the motor abilities of healthy individuals, and drive the development of next-generation robots. Signals from the brain can be captured noninvasively using electroencephalography and processed to control robotic arms (4), (75).
AI applications have the potential to significantly enhance various aspects of neurosurgical practice, including diagnostics, prognostication, decision-making, and data management. This can improve patient safety and surgical education. It is crucial to thoroughly understand and address the potential risks, limitations, and challenges associated with AI before its widespread adoption. Future clinicians must remain informed about the advancements in health care and be capable of integrating these innovations into their practices to achieve better outcomes.
None
This work was supported by JSPS KAKENHI grant number JP21K09091.
We would like to thank Editage (www.editage.com) for its English language editing.
T.S. contributed to study conception and design. Data were collected and analyzed by T.S., H.S., and M.T. Drafts of the manuscript were written by T.S. All authors have read and approved the final manuscript. T.S. contributed to the grant acquisition. M.F. contributed to study supervision.
This study was approved by the institutional review board of Hokkaido University Hospital, Sapporo, Japan (No. 018-0291).
Miki Fujimura is one of the Editors of JMA Journal and on the journal’s Editorial Staff. He was not involved in the editorial evaluation or decision to accept this article for publication at all.
Sugiyama T, Lama S, Hoshyarmanesh H, et al. Early developments, current systems, and future directions. New York: Humana, NY; 2021. Neurosurgical robotics. Neuromethods, vol 162; p. 193-227.
Kennedy-Metz LR, Mascagni P, Torralba A, et al. Computer vision in the operating room: opportunities and caveats. IEEE Trans Med Robot Bionics. 2021;3(1):2-10.
Howell AM, Panesar SS, Burns EM, et al. Reducing the burden of surgical harm: a systematic review of the interventions used to reduce adverse events in surgery. Ann Surg. 2014;259(4):630-41.
Mofatteh M. Neurosurgery and artificial intelligence. AIMS Neurosci. 2021;8(4):477-95.
Houkin K, Baba T, Minamida Y, et al. Quantitative analysis of adverse events in neurosurgery. Neurosurgery. 2009;65(3):587-94.
Stone S, Bernstein M. Prospective error recording in surgery: an analysis of 1108 elective neurosurgical cases. Neurosurgery. 2007;60(6):1075-82.
Rolston J, Zygourakis C, Han S, et al. Medical errors in neurosurgery. Surg Neurol Int. 2014;5(10Supplement):S435-9.
Panesar SS, Kliot M, Parrish R, et al. Promises and perils of artificial intelligence in neurosurgery. Neurosurgery. 2020;87(1):33-44.
Zlochower A, Chow DS, Chang P, et al. Deep learning AI applications in the imaging of glioma. Top Magn Reson Imaging. 2020;29(2):115-21.
Buchlak QD, Esmaili N, Leveque JC, et al. Machine learning applications to neuroimaging for glioma detection and classification: an artificial intelligence augmented systematic review. J Clin Neurosci. 2021;89:177-98.
Li Z, Wang Y, Yu J, et al. Deep learning based radiomics (DLR) and its usage in noninvasive IDH1 prediction for low grade glioma. Sci Rep. 2017;7(1):5467.
Chang P, Grinband J, Weinberg BD, et al. Deep-learning convolutional neural networks accurately classify genetic mutations in gliomas. Am J Neuroradiol. 2018;39(7):1201-7.
Han L, Kamdar MR. MRI to MGMT: predicting methylation status in glioblastoma patients using convolutional recurrent neural networks. World Scientific Publishing Co. Pte Ltd; 2018. Pacific symposium on biocomputing; p. 331-42.
Zhou H, Chang K, Bai HX, et al. Machine learning reveals multimodal MRI patterns predictive of isocitrate dehydrogenase and 1p/19q status in diffuse low- and high-grade gliomas. J Neurooncol. 2019;142(2):299-307.
Yan J, Liu L, Wang W, et al. Radiomic features from multi-parameter MRI combined with clinical parameters predict molecular subgroups in patients with medulloblastoma. Front Oncol. 2020;10:558162.
Park A, Chute C, Rajpurkar P, et al. Deep learning-assisted diagnosis of cerebral aneurysms using the HeadXNet Model. JAMA Netw Open. 2019;2(6):e195600.
Zhu W, Li W, Tian Z, et al. Stability assessment of intracranial aneurysms using machine learning based on clinical and morphological features. Transl Stroke Res. 2020;11(6):1287-95.
Liu Q, Jiang P, Jiang Y, et al. Prediction of aneurysm stability using a machine learning model based on PyRadiomics-derived morphological features. Stroke. 2019;50(9):2314-21.
Han K, Liu C, Friedman D. Artificial intelligence/machine learning for epilepsy and seizure diagnosis. Epilepsy Behav. 2024;155:109736.
Awuah WA, Adebusoye FT, Wellington J, et al. Recent outcomes and challenges of artificial intelligence, machine learning, and deep learning in neurosurgery. World Neurosurg. 2024;23:100301.
Tangsrivimol JA, Schonfeld E, Zhang M, et al. Artificial intelligence in neurosurgery: a state-of-the-art review from past to future. Diagnostics. 2023;13(14):2429.
Van Niftrik CHB, Van Der Wouden F, Staartjes VE, et al. Machine learning algorithm identifies patients at high risk for early complications after intracranial tumor surgery: registry-based cohort study. Clin Neurosurg. 2019;85(4):E756-64.
Durand WM, Depasse JM, Daniels AH. Predictive modeling for blood transfusion after adult spinal deformity surgery. Spine (Phila Pa 1976). 2018;43(15):1058-66.
Tewarie IA, Senko AW, Jessurun CAC, et al. Predicting leptomeningeal disease spread after resection of brain metastases using machine learning. J Neurosurg. 2022;138(6):1561-9.
Larivière S, Weng Y, Vos de Wael R, et al. Functional connectome contractions in temporal lobe epilepsy: microstructural underpinnings and predictors of surgical outcome. Epilepsia. 2020;61(6):1221-33.
Ames CP, Smith JS, Pellisé F, et al. Artificial intelligence based hierarchical clustering of patient types and intervention categories in adult spinal deformity surgery: towards a new classification scheme that predicts quality and value. Spine (Phila Pa 1976). 2019;44(13):915-26.
Ramos LA, Van Der Steen WE, Sales Barros R, et al. Machine learning improves prediction of delayed cerebral ischemia in patients with subarachnoid hemorrhage. J Neurointerv Surg. 2019;11(5):497-502.
Koch M, Acharjee A, Ament Z, et al. Machine learning-driven metabolomic evaluation of cerebrospinal fluid: insights into poor outcomes after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2021;88(5):1003-11.
Paliwal N, Jaiswal P, Tutino VM, et al. Outcome prediction of intracranial aneurysm treatment by flow diverters using machine learning. Neurosurg Focus. 2018;45(5):E7.
Warman A, Kalluri AL, Azad TD. Machine learning predictive models in neurosurgery: an appraisal based on the TRIPOD guidelines. Systematic review. Neurosurg Focus. 2023;54(6):E8.
Huang J, Shlobin NA, Decuypere M, et al. Deep learning for outcome prediction in neurosurgery: a systematic review of design, reporting, and reproducibility. Neurosurgery. 2022;90(1):16-38.
Shen B, Zhang Z, Shi X, et al. Real-time intraoperative glioma diagnosis using fluorescence imaging and deep convolutional neural networks. Eur J Nucl Med Mol Imaging. 2021;48(11):3482-92.
Hollon T, Orringer DA. Label-free brain tumor imaging using Raman-based methods. J Neurooncol. 2021;151(3):393-402.
Cakmakci D, Karakaslar EO, Ruhland E, et al. Machine learning assisted intraoperative assessment of brain tumor margins using HRMAS NMR spectroscopy. PLoS Comput Biol. 2020;16(11):e1008184.
Qiao N, Song M, Ye Z, et al. Deep learning for automatically visual evoked potential classification during surgical decompression of sellar region tumors. Transl Vis Sci Technol. 2019;8(6):21.
Dundar TT, Yurtsever I, Pehlivanoglu MK, et al. Machine learning-based surgical planning for neurosurgery: artificial intelligent approaches to the cranium. Front Surg. 2022;9:863633.
Tonutti M, Gras G, Yang GZ. A machine learning approach for real-time modelling of tissue deformation in image-guided neurosurgery. Artif Intell Med. 2017;80:39-47.
Phellan R, Hachem B, Clin J, et al. Real-time biomechanics using the finite element method and machine learning: review and perspective. Med Phys. 2021;48(1):7-18.
Khan DZ, Luengo I, Barbarisi S, et al. Automated operative workflow analysis of endoscopic pituitary surgery using machine learning: development and preclinical evaluation (IDEAL stage 0). J Neurosurg. 2022;137(1):51-8.
Ward TM, Mascagni P, Madani A, et al. Surgical data science and artificial intelligence for surgical education. J Surg Oncol. 2021;124(2):221-30.
Maier-Hein L, Eisenmann M, Sarikaya D, et al. Surgical data science - from concepts toward clinical translation. Med Image Anal. 2022;76:102306.
Fecso AB, Szasz P, Kerezov G, et al. The effect of technical performance on patient outcomes in surgery. Ann Surg. 2017;265(3):492-501.
Sugiyama T, Lama S, Gan LS, et al. Forces of tool-tissue interaction to assess surgical skill level. JAMA Surg. 2018;153(3):234-42.
Sugiyama T, Gan LS, Zareinia K, et al. Tool-tissue interaction forces in brain arteriovenous malformation surgery. World Neurosurg. 2017;102:221-8.
Ghasemloonia A, Maddahi Y, Zareinia K, et al. Surgical skill assessment using motion quality and smoothness. J Surg Educ. 2017;74(2):295-305.
Baghdadi A, Guo E, Lama S, et al. Force profile as surgeon-specific signature. Ann Surg Open. 2023;4(3):e326.
Baghdadi A, Lama S, Singh R, et al. Tool-tissue force segmentation and pattern recognition for evaluating neurosurgical performance. Sci Rep. 2023;13(1):9591.
Titov O, Bykanov A, Pitskhelauri D. Neurosurgical skills analysis by machine learning models: systematic review. Neurosurg Rev. 2023;46(1):121.
Mirchi N, Bissonnette V, Yilmaz R, et al. The virtual operative assistant: an explainable artificial intelligence tool for simulation-based training in surgery and medicine. PLoS One. 2020;15(2):e0229596.
Sugiyama T, Clapp T, Nelson J, et al. Immersive 3-dimensional virtual reality modeling for case-specific presurgical discussions in cerebrovascular neurosurgery. Oper Neurosurg. 2021;20(3):289-99.
Sarkiss CA, Philemond S, Lee J, et al. Neurosurgical skills assessment: measuring technical proficiency in neurosurgery residents through intraoperative video evaluations. World Neurosurg. 2016;89:1-8.
Sugimori H, Sugiyama T, Nakayama N, et al. Development of a deep learning-based algorithm to detect the distal end of a surgical instrument. Appl Sci. 2020;10(12):4245.
Pangal DJ, Kugener G, Cardinal T, et al. Use of surgical video-based automated performance metrics to predict blood loss and success of simulated vascular injury control in neurosurgery: a pilot study. J Neurosurg. 2021;137(3):840-9.
Sugiyama T, Ito M, Sugimori H, et al. Tissue acceleration as a novel metric for surgical performance during carotid endarterectomy. Oper Neurosurg. 2023;25(4):343-52.
Sugiyama T, Nakamura T, Ito Y, et al. A pilot study on measuring tissue motion during carotid surgery using video-based analyses for the objective assessment of surgical performance. World J Surg. 2019;43(9):2309-19.
Gonzalez-Romo NI, Hanalioglu S, Mignucci-Jiménez G, et al. Quantification of motion during microvascular anastomosis simulation using machine learning hand detection. Neurosurg Focus. 2023;54(6):E2.
Sugiyama T, Sugimori H, Tang M, et al. Deep learning-based video-analysis of instrument motion in microvascular anastomosis training. Acta Neurochir (Wien). 2024;166(1):6.
Davids J, Makariou SG, Ashrafian H, et al. Automated vision-based microsurgical skill analysis in neurosurgery using deep learning: development and preclinical validation. World Neurosurg. 2021;149:e669-86.
Tang M, Sugiyama T, Takahari R, et al. Assessment of changes in vessel area during needle manipulation in microvascular anastomosis using a deep learning-based semantic segmentation algorithm: a pilot study. Neurosurg Rev. 2024;47(1):200.
Thirunavukarasu AJ, Ting DSJ, Elangovan K, et al. Large language models in medicine. Nat Med. 2023;29(8):1930-40.
Gilson A, Safranek CW, Huang T, et al. How does ChatGPT perform on the United States medical licensing examination? The implications of large language models for medical education and knowledge assessment. JMIR Med Educ. 2023;9:e48305.
Hopkins BS, Nguyen VN, Dallas J, et al. ChatGPT versus the neurosurgical written boards: a comparative analysis of artificial intelligence/machine learning performance on neurosurgical board-style questions. J Neurosurg. 2023;139(3):904-11.
Ali R, Tang OY, Connolly ID, et al. Performance of ChatGPT, GPT-4, and Google Bard on a neurosurgery oral boards preparation question bank. Neurosurgery. 2023;93(5):1090-8.
O’Malley GR, Sarwar SA, Cassimatis ND, et al. Can publicly available artificial intelligence successfully identify current procedural terminology codes for common procedures in neurosurgery? World Neurosurg. 2024;183:e860-70.
Horiuchi D, Tatekawa H, Shimono T, et al. Accuracy of ChatGPT generated diagnosis from patient’s medical history and imaging findings in neuroradiology cases. Neuroradiology. 2024;66(1):73-9.
Mishra A, Begley SL, Chen A, et al. Exploring the intersection of artificial intelligence and neurosurgery: let us be cautious with ChatGPT. Neurosurgery. 2023;93(6):1366-73.
Huang KT, Mehta NH, Gupta S, et al. Evaluation of the safety, accuracy, and helpfulness of the GPT-4.0 Large Language Model in neurosurgery. J Clin Neurosci. 2024;123:151-6.
Ward M, Unadkat P, Toscano D, et al. A quantitative assessment of ChatGPT as a neurosurgical triaging tool. Neurosurgery. 2024;95(2):487-95.
Gajjar AA, Kumar RP, Paliwoda ED, et al. Usefulness and accuracy of artificial intelligence Chatbot responses to patient questions for neurosurgical procedures. Neurosurgery. Forthcoming 2024. Available from: https://pubmed.ncbi.nlm.nih.gov/38353558/
Guo E, Gupta M, Sinha S, et al. neuroGPT-X: toward a clinic-ready large language model. J Neurosurg. 2023;140(4):1041-53.
Murff HJ, FitzHenry F, Matheny ME, et al. Automated identification of postoperative complications within an electronic medical record using natural language processing. JAMA. 2011;306(8):848-55.
Thirukumaran CP, Zaman A, Rubery PT, et al. Natural language processing for the identification of surgical site infections in prthopaedics. J Bone Jt Surg Am. 2019;101(24):2167-74.
Lundberg SM, Nair B, Vavilala MS, et al. Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat Biomed Eng. 2018;2(10):749-60.
Fazlollahi AM, Bakhaidar M, Alsayegh A, et al. Effect of artificial intelligence tutoring vs expert instruction on learning simulated surgical skills among medical students a randomized clinical trial. JAMA Netw Open. 2022;5(2):e2149008.
Zhang X, Ma Z, Zheng H, et al. The combination of brain-computer interfaces and artificial intelligence: applications and challenges. Ann Transl Med. 2020;8(11):712.