Corresponding author: Yasuaki Harabuchi, hyasu@asahikawa-med.ac.jp
DOI: 10.31662/jmaj.2024-0437
Received: January 13, 2025
Accepted: January 28, 2025
Advance Publication: March 21, 2025
Published: April 28, 2025
Cite this article as:
Harabuchi Y, Kumai T, Nishi K, Tanaka A, Hotta O, Hagino H, Kusuyama T, Mogitate M, Ohno Y, Sakakibara A, Araki S, Nishida Y, Shintani T, Takezawa H, Ito H, Komazawa D, Nishiwaki N, Toritani R, Hirahata K, Marumo S. Retracted: Chronic Epipharyngitis Treated with Epipharyngeal Abrasion Therapy: Symptoms, Diagnosis, Pathogenesis, and Treatment Outcomes. JMA J. 2025;8(2):371-384.
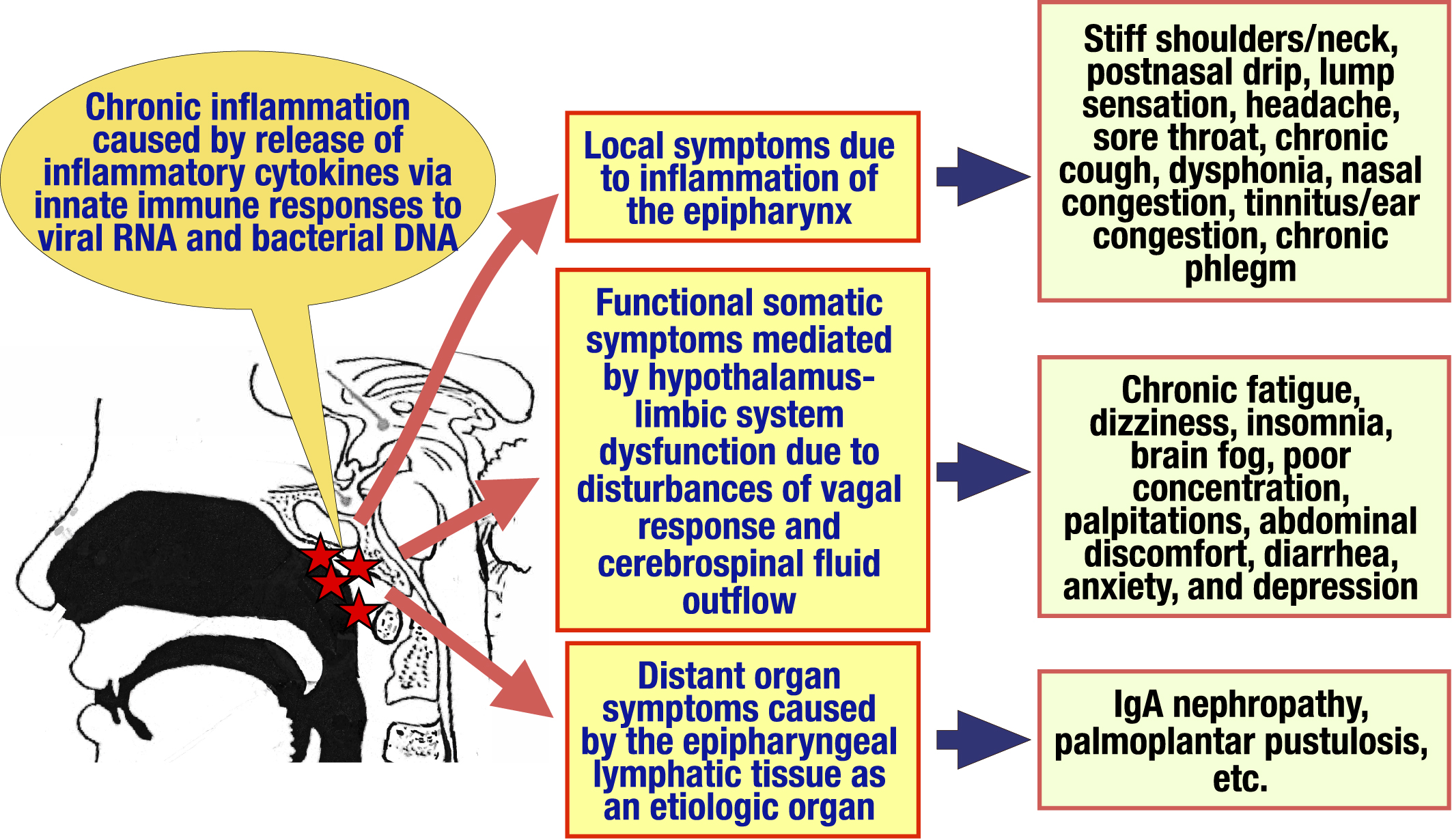
Chronic epipharyngitis is associated with a wide variety of symptoms, including local symptoms such as postnasal drip, sore throat, lump sensation of the pharynx, headache, chronic cough, nasal obstruction, tinnitus/ear fullness, chronic phlegm and dysphonia due to inflammation of the epipharynx, functional somatic symptoms such as chronic fatigue, dizziness, insomnia, brain fog, abdominal discomfort, and depression caused by dysfunction of the hypothalamus-limbic system via disturbances of vagal response and cerebrospinal fluid outflow, and distant organ symptoms such as immunoglobulin A nephropathy and palmoplantar pustulosis caused by the epipharyngeal lymphoid tissue as an etiologic organ. In the past, chronic inflammation in the epipharynx was difficult to prove by gross findings, now, direct observation of the epipharyngeal inflammation by endoscopy has become easier for the diagnosis. For the treatment of chronic epipharyngitis, epipharyngeal abrasive therapy (EAT), epipharyngeal application of a 1% zinc chloride solution intranasally or orally was popular since the 1960s, recently, endoscopic EAT (E-EAT), in which epipharynx is safely and accurately observed and abraded under clear vision using an endoscope, has been developed. The mechanisms of EAT effects can be classified into anti-inflammatory/antiviral effect, bloodletting effect, and vagus nerve stimulation effect. Recently, the effectiveness of EAT for post-acute sequelae of coronavirus disease 2019 (COVID-19), known as long COVID, has come into the limelight, and the number of patients for whom EAT is expected to increase. In 2019, the Japan Society of Stomato-pharyngology established the EAT Review Committee to accumulate evidence on the efficacy of EAT and to establish indications and techniques for its use. In this article, the EAT Review Committee outlines its symptoms, pathogenesis, and diagnosis of chronic epipharyngitis, technique of E-EAT, mechanisms of EAT effects, past reports for the efficacy of EAT, and a multicenter prospective study.
Key words: chronic epipharyngitis, epipharyngeal abrasive therapy (EAT), long COVID, myalgia encephalomyelitis/chronic fatigue syndrome, vagus nerve stimulation autoimmune/inflammatory syndrome induced by adjuvants (ASIA), IgA nephropathy, palmoplantar pustulosis
In Japan, chronic epipharyngitis has been known to cause many complaints since the 1930s. In the 1960s and 1970s, Yamazaki (1), (2) and Horiguchi (3), (4), (5) reported that the symptoms of chronic epipharyngitis were not limited to those caused by local inflammation of the epipharynx but also included headache, brain fog, stiff shoulders, dizziness, general fatigue, sleep disorder, gastric discomfort, irregular bowel movements, depression, and other functional somatic symptoms. Horiguchi (3), (4), (5) demonstrated that the treatment with 1% zinc chloride solution applied intranasally or orally to the epipharynx was extremely effective, and then, this treatment method became widely used in Japan.
Later, in the 2010s, the name for the treatment method proposed by Horiguchi came to be called “epipharyngeal abrasive therapy (EAT)” (6) as an accurate and meaning-clear term. Then, instead of the conventional blind procedure, endoscopic EAT (E-EAT), in which the epipharynx is safely and accurately observed and abraded under clear vision using an endoscope, was developed (7) and its effectiveness began to attract attention.
In 2013, the Japanese Focal Inflammation-Related Disease Research Group (https://jfir.jp/) was established to educate the general public, and many special lectures, public lectures, and general presentations on EAT are given in the annual meeting every year. Furthermore, EAT has attracted attention not only in Japan but also worldwide as an effective treatment for post-acute sequelae of coronavirus disease 2019 (COVID-19), known as long COVID, which occurs in 10%-20% of COVID-19 patients (8), (9), and has been publicly reported on NHK WORLD (10), etc. Based on this background, in 2019, the EAT Review Committee was established in the Japan Society of Stomato-pharyngology. In this paper, the Committee outlines diagnostic criteria, symptoms, and pathogenesis of chronic epipharyngitis as well as techniques of E-EAT, the effects of EAT, and a multicenter prospective study for EAT.
In the past, chronic epipharyngitis was not well-defined as a disease. Horiguchi (11), (12), (13) stated that “the diagnosis of chronic epipharyngitis is difficult to determine by gross findings, and that a smear obtained by rubbing the epipharynx and the pain and bleeding from mucous membranes by abrasion is useful to determine the disease”. However, cytological diagnosis by swab is not reproducible, and the pain by the abrasion varies from person to person and lacks objectivity, limiting the widespread use of the diagnostic criteria.
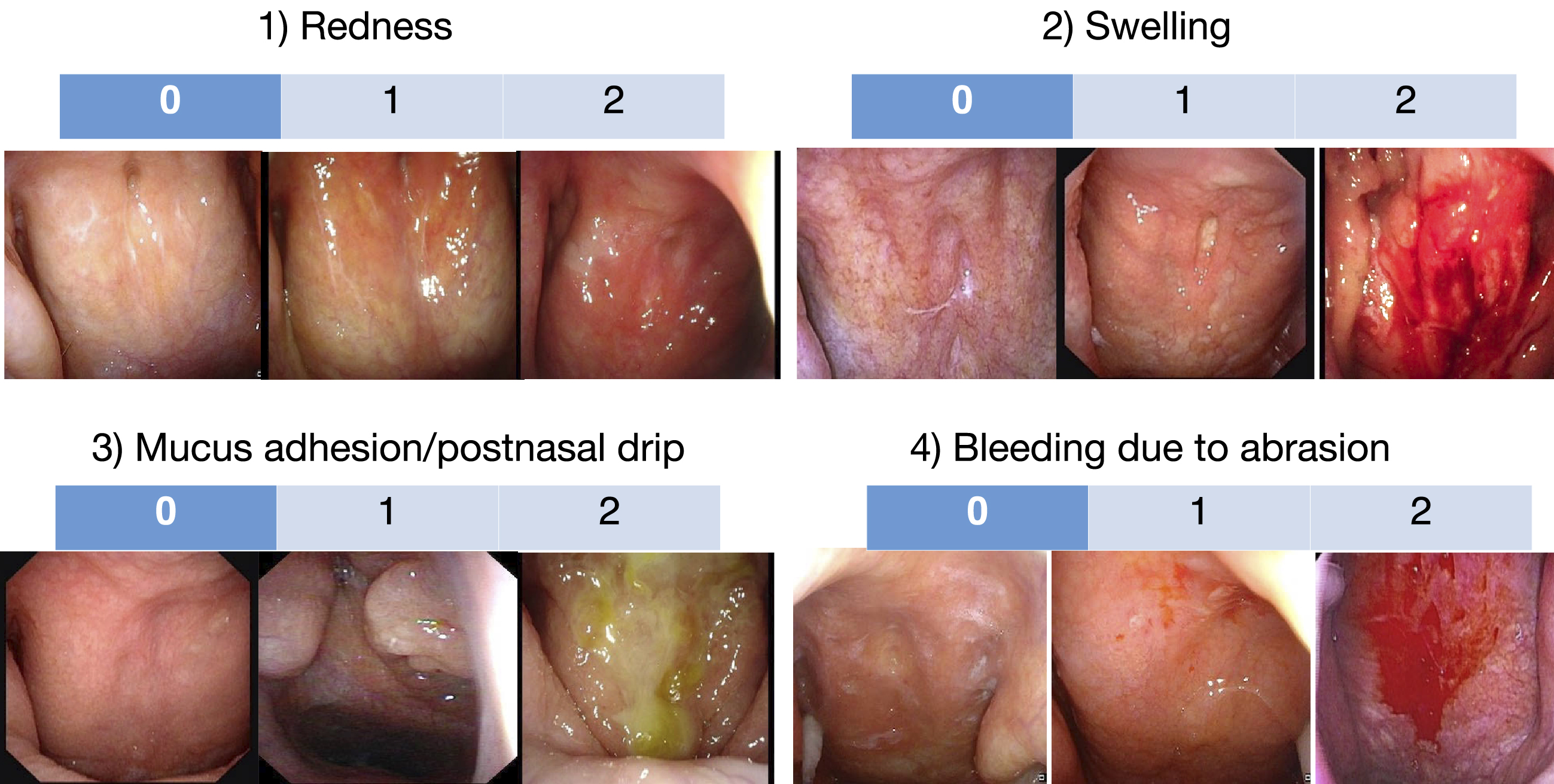
Now that direct observation of epipharyngeal findings by endoscopy has become easier, the EAT Review Committee proposed the diagnostic criteria for this disease as follows (Table 1); “All 3 of the following must be met: (1) Symptoms suggestive of chronic epipharyngitis that do not improve with any medical treatment persist for at least one month; (2) No organic disease of the nasal cavity, paranasal sinuses, oropharynx, hypopharynx, or larynx, (3) Endoscopic evidence of inflammation in the epipharynx such as redness, swelling of the mucous membrane and adhesion/postnasal drip of epipharyngeal-derived mucus, in addition to observation of bleeding due to epipharyngeal abrasion”. In addition, the EAT Review Committee proposed that the severity of 4 epipharyngeal endoscopic findings, (1) redness, (2) swelling, (3) mucus adhesion/postnasal drip, and (4) bleeding due to abrasion, are scored on a three-point scale from 0 to 2, respectively, and the sum of these scores are used as an index of epipharyngeal inflammation (Figure 1).
Table 1. Diagnostic Criteria for Chronic Epipharyngitis.
| All 3 of the following criteria must be met |
|---|
| (1) Symptoms suggestive of chronic epipharyngitis that do not improve with any medical treatment persist for at least one month |
| (2) No organic disease of the nasal cavity, paranasal sinuses, oropharynx, hypopharynx, or larynx |
| (3) Endoscopic evidence of inflammation in the epipharynx such as redness, swelling of the mucous membrane and adhesion/postnasal drip of epipharyngeal-derived mucus, in addition to bleeding due to epipharyngeal abrasion |
Inflammation in chronic epipharyngitis is not necessarily the result of acute bacterial or viral infections that have become chronic. The epipharyngeal lymphoid tissue at the entrance to the respiratory tract is the primary immune responder against various antigens (14). Innate immune ligands such as viral ribonucleic acid (RNA), bacterial deoxyribonucleic acid (DNA), and aluminum immunostimulatory adjuvants of human papillomavirus (HPV) and novel coronavirus vaccines stimulate lymphoid tissues, causing chronic inflammation of the epipharyngeal tissues through the release of inflammatory cytokines, resulting in the appearance of various symptoms (Table 2 and Figure 2). Thus, chronic epipharyngitis can be categorized as “adjuvant-induced autoimmune inflammatory syndrome caused by adjuvant substances that stimulate the immune system (ASIA)” (15). Symptoms and pathogenesis of chronic epipharyngitis are classified into the following 3 categories.
Table 2. Appearance rates* of the Symptoms in Chronic Epipharyngitis.
| Local symptoms due to inflammation of the epipharynx | Functional somatic symptoms due to hypothalamus-limbic system dysfunction through disturbances of vagal response and cerebrospinal fluid outflow | Distant organ symptoms caused by the epipharynx lymphatic tissue as an etiologic organ | |||
|---|---|---|---|---|---|
| Stiff shoulders/stiff neck | 89 | Chronic fatigue | 86 | Sternocostal joint pain | 4 |
| Postnasal drip | 72 | Dizzy sensation | 46 | Psoriasis | 1 |
| Pharyngeal discomfort | 72 | Insomnia | 10 | Chronic urticaria | 1 |
| Headache | 63 | Brain fog | 10 | ||
| Sore throat | 46 | Difficulty breathing | 9 | ||
| Chronic cough | 39 | Photophobia | 6 | ||
| Voice disorder | 39 | Lower back pain/back pain | 3 | ||
| Olfaction/Taste disorder | 8 | Palpitations | 4 | ||
| Nasal congestion | 6 | Slight fever | 2 | ||
| Tinnitus/Ear congestion | 5 | Restless legs | 2 | ||
| Chronic phlegm | 5 | Tongue pain | 2 | ||
| Facial discomfort | 3 | Nausea | 2 | ||
| Toothache | 1 | Whole body neuralgia | 2 | ||
| Nose pain | 1 | Anxiety disorder | 1 | ||
| Snoring | 1 | Numbness in limbs | 1 | ||
| Pain behind the eyes | 1 | Diarrhea/Constipation | 1 | ||
| Gastrointestinal disorders | 1 | ||||
| Hot flashes | 1 | ||||
| Chest pain | 1 | ||||
| Appetite loss | 1 | ||||
| *Appearnce rate rates are expressed as the percentage of patients who complained the symptom among 130 patients enrolled for an interim analysis of the multicenter prospective study for effecacy of EAT. | |||||
In 130 patients enrolled for an interim analysis of the multicenter prospective study conducted by the EAT Review Committee, 60%-80% of patients had symptoms of stiff shoulders/stiff neck, postnasal drip, pharyngeal discomfort, and headache; 30%-40% had sore throat, chronic cough, and dysphonia; and 5%-10% had olfactory/taste disorders, nasal congestion, tinnitus/ear congestion, and chronic phlegm (Table 2).
Postnasal drip is secreted via histamine and other chemical mediators released by the activation of the immune response of the epipharyngeal lymphoid tissue. Irritation of postnasal drip causes pharyngeal discomfort, sore throat, and chronic cough. Nasal congestion, olfactory/taste disorders, tinnitus/ear congestion, and voice disorders are caused by obstruction of the epipharynx due to mucosal edema and/or hypertrophy of the pharyngeal tonsils. Headaches are often caused by excessive stimulation of the pterygopalatine ganglion via the second branch of the trigeminal nerve, which distributes the epipharynx, in the same mechanism as Sluder’s neuralgia (16). Stiff shoulder/stiff neck may also occur through the same mechanism as referred pain, but may also involve mechanisms mediated by hypothalamus-limbic system dysfunction.
As shown in Table 1, patients with chronic epipharyngitis often complain of symptoms such as chronic fatigue, dizziness, insomnia, brain fog, poor concentration, palpitations, abdominal discomfort, diarrhea, anxiety, and depression. These symptoms are called “functional somatic symptoms” because routine endoscopic, imaging, and physiologic tests are normal and do not clearly explain the presence of organic disease. Functional somatic symptoms are also seen in a variety of diseases, including fibromyalgia, chronic fatigue syndrome, HPV vaccine sequelae, and novel coronavirus infection sequelae (long COVID). Recently, it has been suggested that the etiology of such symptoms involves hypothalamus-limbic system dysfunction due to disturbances of vagal response and cerebrospinal fluid outflow (6).
The vagal response is known to have anti-inflammatory effects through the cholinergic anti-inflammatory pathway and the solitary tract nucleus (STN)-paraventricular nucleus (PVN)-pituitary-adrenal pathway, and prevents potentially harmful inflammation (17), (18). Tactile and visceral sensations in peripheral tissues stimulate vagal afferent pathways, which activate the STN. The signal is transmitted to the dorsal motor nucleus (DMN) via PVN in the hypothalamus, reaches various organs, and then, inflammation is suppressed by the release of acetylcholine at each organ. In another pathway, the signals transmitted to the vagus afferent tract-STN-PVN activate the pituitary gland, ultimately resulting in the secretion of cortisol from the adrenal gland (17). Furthermore, activation signals transmitted from the STN to the PVN activate the hypothalamus-limbic system via the area postrema, regulating emotion, cognition, and behavior (19). Because the epipharyngeal mucosa contains many vagal afferent fibers (20), (21), (22), epipharyngeal tactile and visceral sensory signals activates also vagal afferent tracts to produce the same effects.
In chronic epipharyngitis, on the other hand, inflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, which are continuously overproduced by chronic inflammation, overstimulate the vagal afferent tract. The resulting vagal response is impaired, leading to inflammation in various organs, decreased cortisol secretion, and hypothalamus-limbic system dysfunction, followed by multi-organ symptoms as well as functional somatic symptoms (Figure 3). In support of this model, vagal response dysfunction and chronic epipharyngitis are extremely frequent in long COVID (23), fibromyalgia, chronic fatigue syndrome, chronic headache, irritable bowel syndrome, and functional dyspepsia (8), (24), (25), (26). Patients with chronic epipharyngitis with these comorbidities show abnormal vagal responses on multidimensional autonomic function tests, and these patients recover on EAT (27), (28), (29), (30).
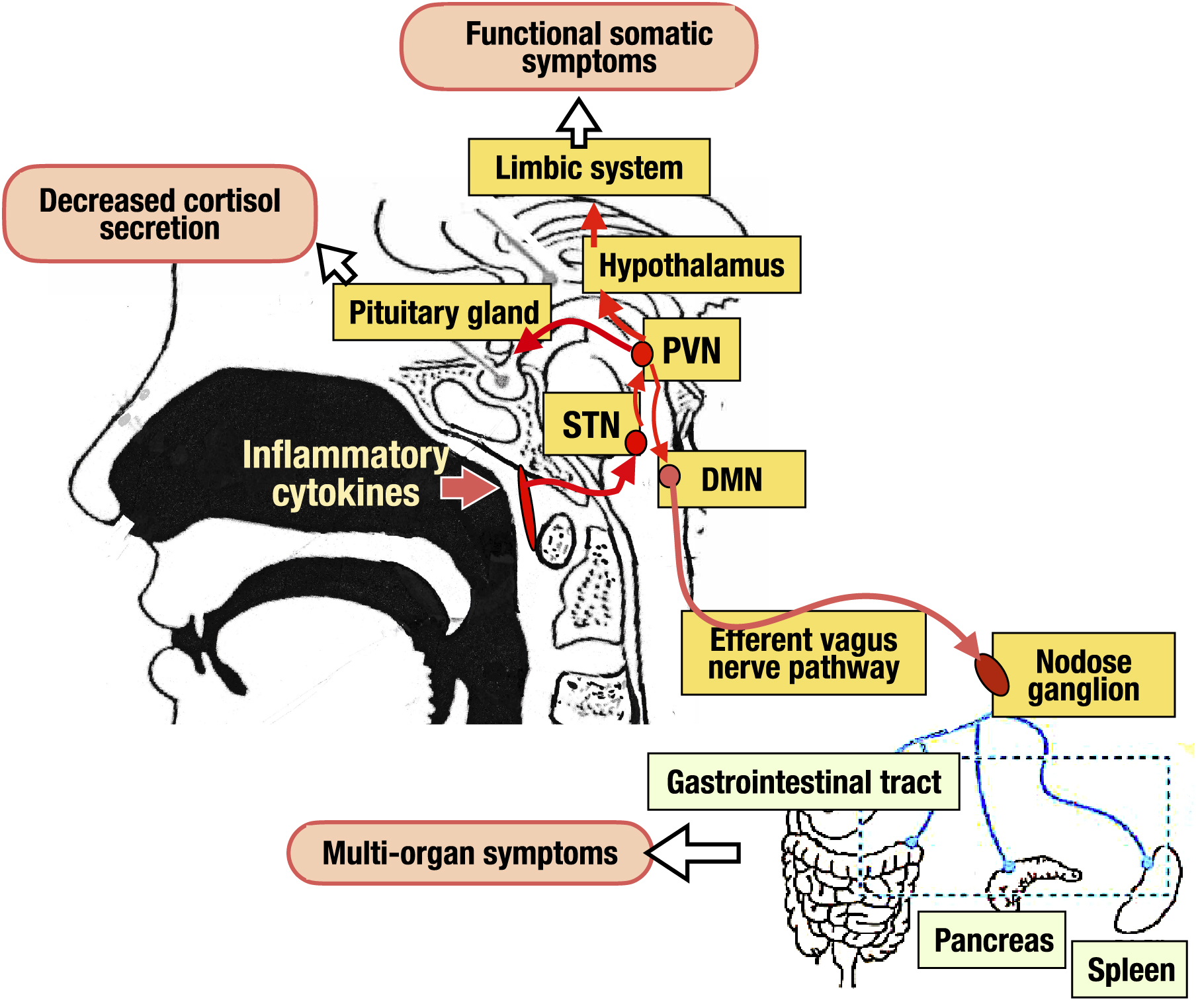
Increased cerebrospinal fluid pressure due to lymphatic stasis is also seen in fibromyalgia, chronic fatigue syndrome, and long COVID and contributes to functional somatic symptoms (31), (32). Cerebrospinal fluid passes through lymphatic vessels in the subarachnoid space, follows through a network of lymphatic vessels lining the mucous membranes of the ethmoid sinus, nasal septum, lateral nasal wall, hard palate, and soft palate, joining the epipharyngeal lymph plexus as a hub and flowing into the cervical lymph nodes (33). Thus, epipharyngeal edema due to chronic epipharyngitis leads to lymphatic stasis and increased cerebrospinal fluid pressure via impaired cerebrospinal fluid outflow. The resulting hypothalamus-limbic system dysfunction is thought to cause various functional somatic symptoms.
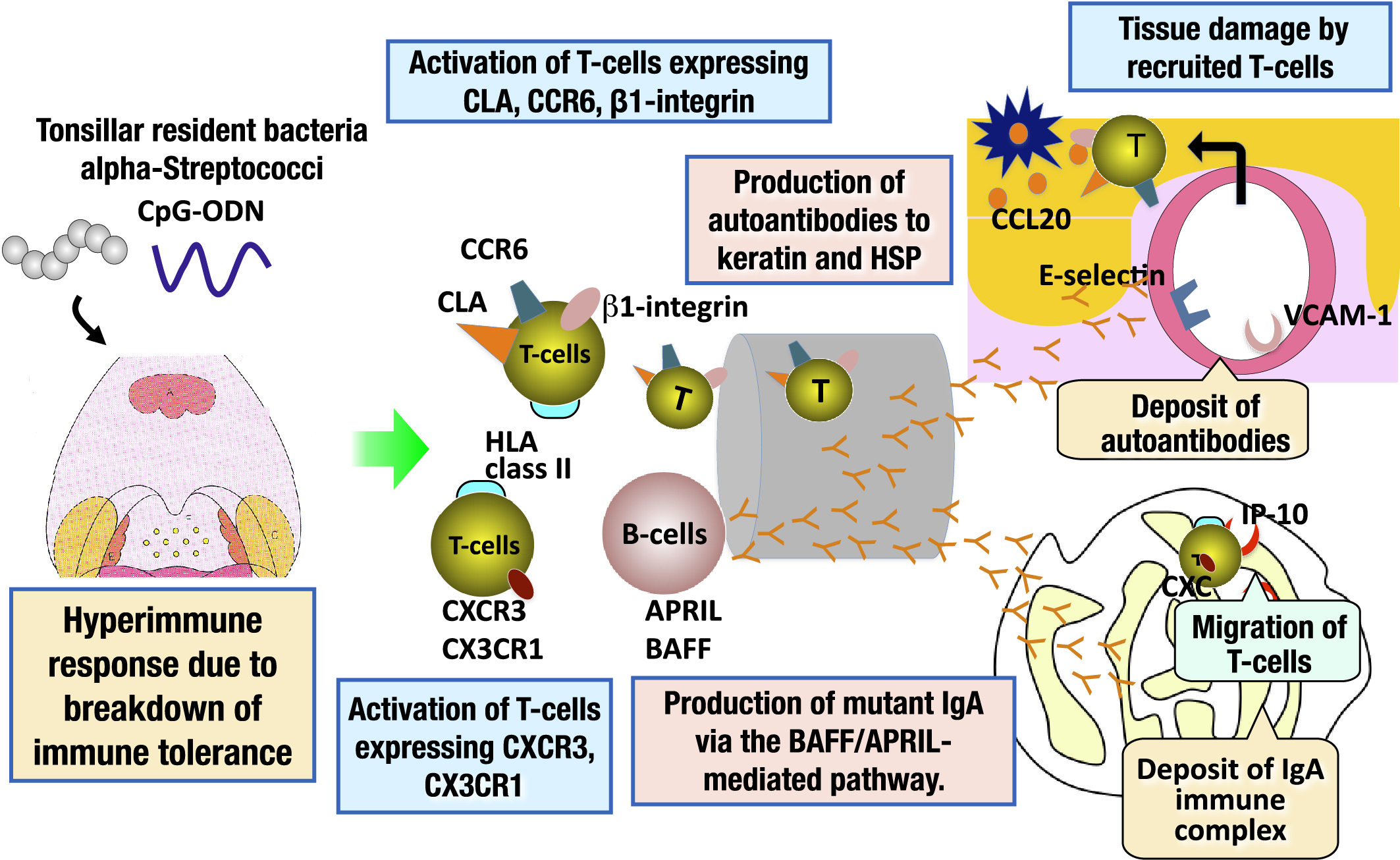
Immunoglobulin A (IgA) nephropathy and palmoplantar pustulosis are categorized as tonsil-induced autoimmune/inflammatory syndrome (TIAS). Patients with TIAS have a breakdown of immune tolerance to tonsillar resident bacteria, resulting in a hyperimmune response to these microorganisms. As a result, tonsillar B-cells are activated and the autoantibodies against common antigens to the skin, and mutant IgA are produced, transported to the skin and renal glomeruli, and deposited in these tissues. In addition, tonsillar T-cells that have an affinity for the skin or renal glomeruli are also activated and home to the skin and kidney, resulting in tissue damage in the target organs (34), (35) (Figure 4). The epipharyngeal lymphoid tissue has the same immune response mechanism as the palatine tonsils (14). Therefore, the epipharyngeal lymphoid tissue of TIAS patients is the etiologic organ of these diseases, as is the palatine tonsil (36), (37), (38).
The EAT Review Committee recommends the transnasal method first followed by the transoral method for E-EAT, using a 1% zinc chloride as the medicinal solution.
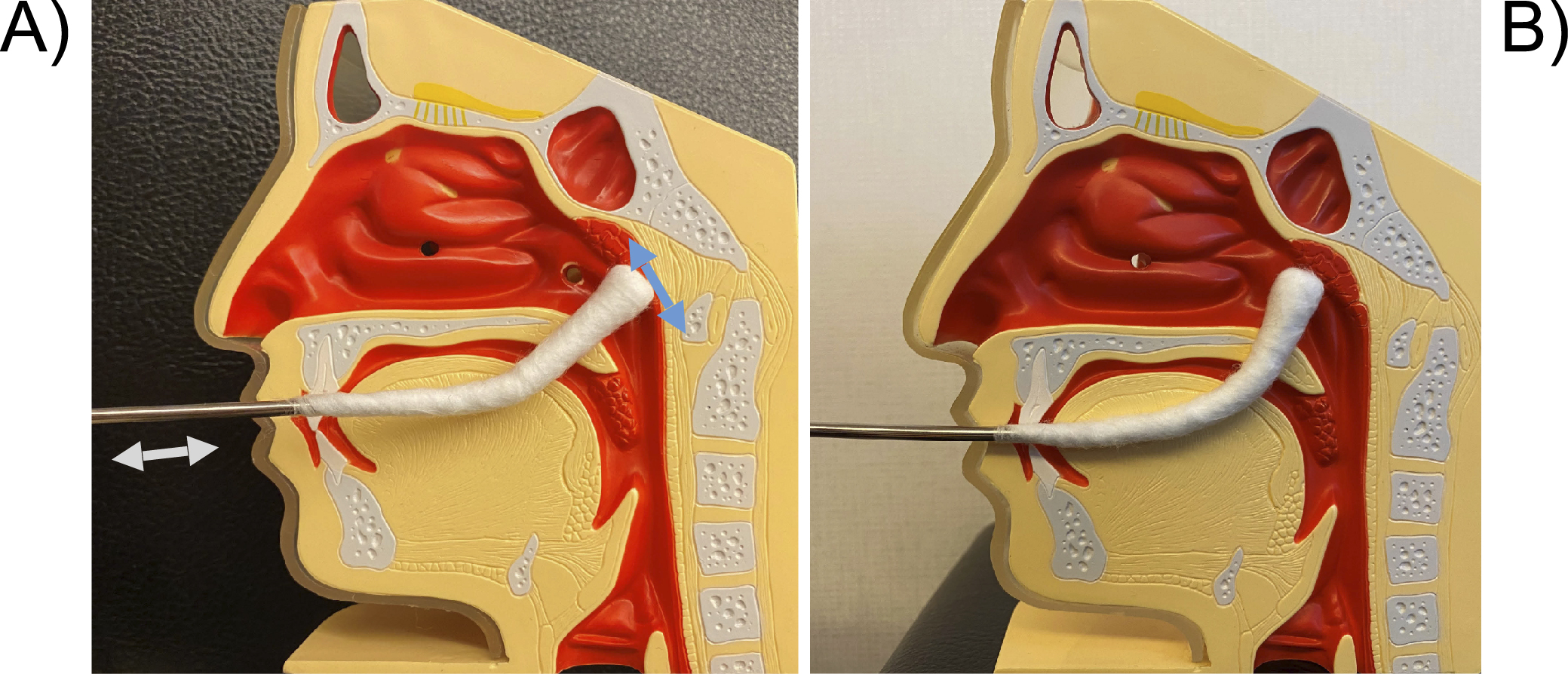
An endoscope is inserted into the right nasal cavity and a cotton applicator is inserted into the left nasal cavity, and a slightly curved nasal cotton applicator is used to widely abrade the mucosa from the midline of the epipharyngeal fossa to the posterior wall in the up, down, left, and right directions. The basic principle is not to “apply” but to “abrade” the mucosa with just enough force to lightly scrape the mucosa, and if there is inflammation, the edematous mucosa can easily bleed or peel off. The applicator is then directed downward to abrade the left and right Rosenmüller’s fossa.
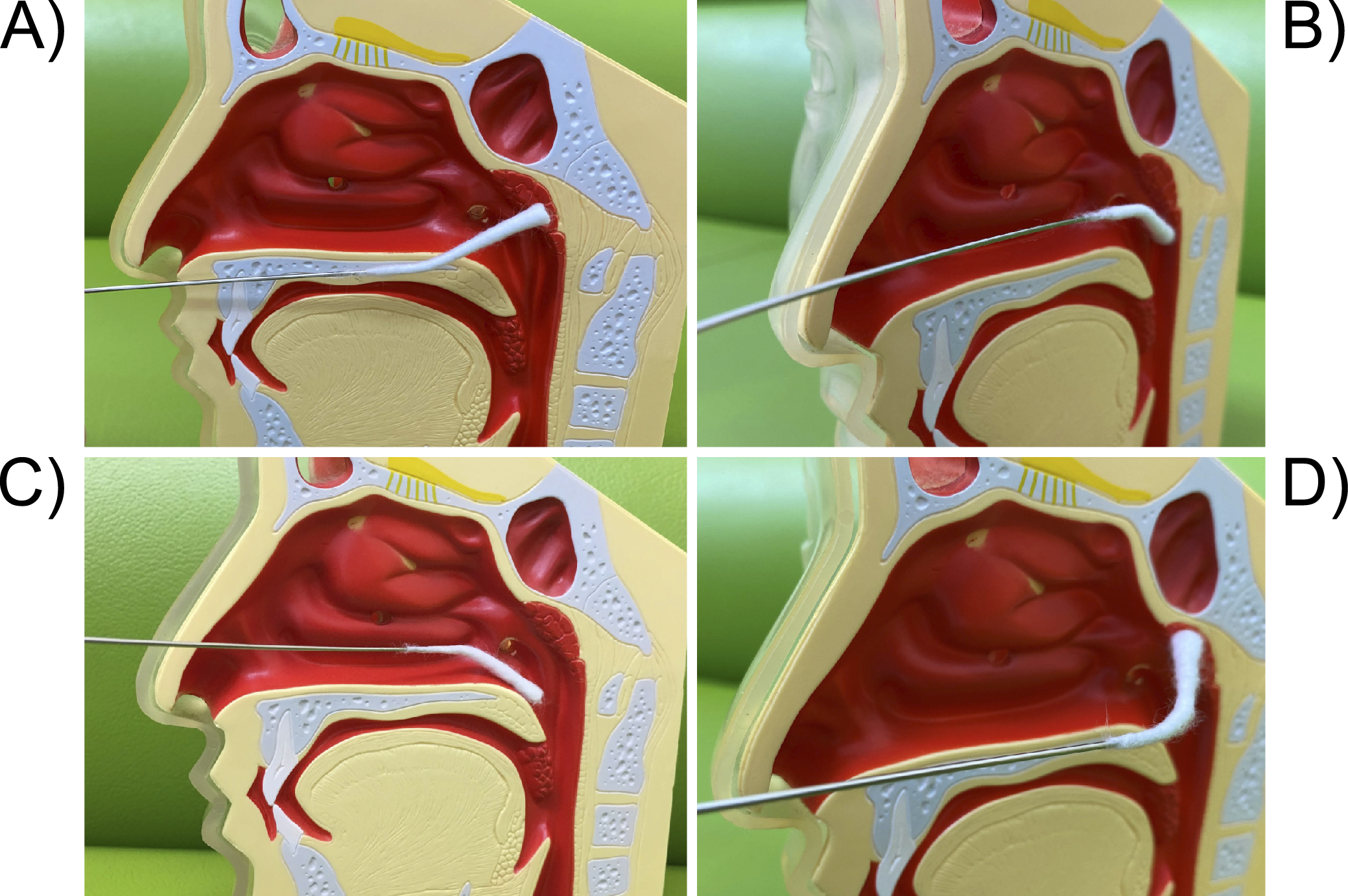
The pharyngeal cotton applicator is inserted horizontally into the oral cavity. When it hits the posterior wall of the oropharynx, it is inverted upward and the cotton applicator is moved in a wiper-like motion from side to side as if it were being rubbed up toward the patient. The entire area of the epipharynx should also be abraded.
EAT effects are broadly composed of 3 categories: 1) anti-inflammatory/antiviral effect, 2) bloodletting effect, and 3) vagus nerve stimulation (VNS) effect (Figure 7) (44). The following reports show these effects.
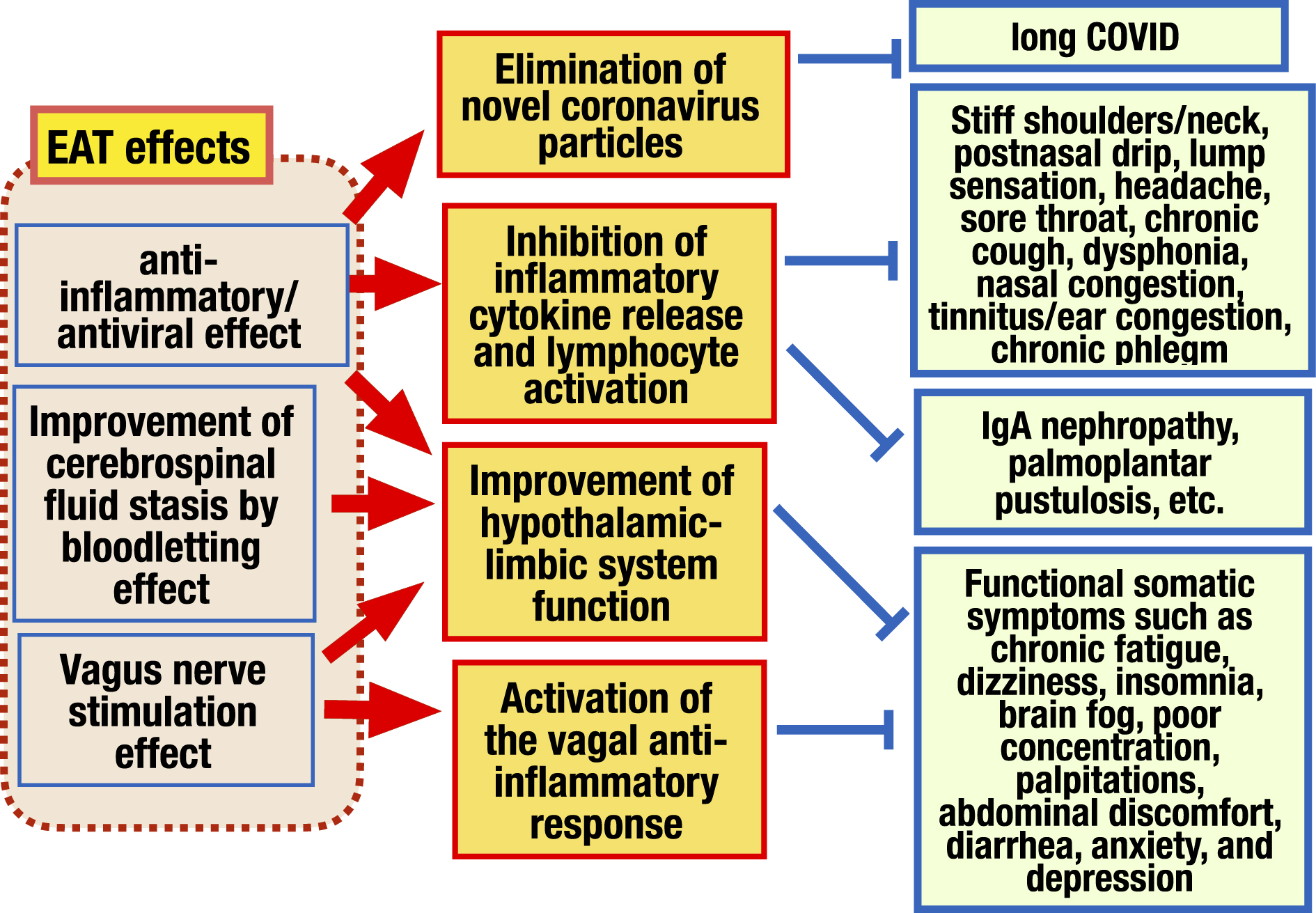
Mogitate (45), (46) measured the exhaled nitric oxide (NO) concentration before and after EAT at the initial visit and follow-up in 102 patients with chronic epipharyngitis. As a result, the severity of endoscopic findings and the exhaled NO concentration showed a positive correlation, and the continued EAT reduced the exhaled NO concentration along with the recovery of symptoms and endoscopic findings, suggesting that EAT has an anti-inflammatory effect and that the exhaled NO concentration reflects the severity of epipharyngitis. He found also that EAT reduced exhaled NO concentration regardless of the presence or absence of zinc chloride, suggesting that EAT without zinc chloride also has a therapeutic effect, and zinc chloride enhances this effect.
Nishi et al. (47), (48), (49), (50) have demonstrated the anti-inflammatory/antiviral effect of EAT by histological analysis of epipharyngeal tissue. They (47) analyzed the epipharyngeal mucosa of patients with chronic epipharyngitis using in situ hybridization and demonstrated that IL-6 and TNFα messenger RNA (mRNA) expressions in 11 patients with EAT treatment were significantly lower than that in 6 patients without EAT, indicating that EAT has the effect of suppressing inflammatory cytokine production. In addition, they demonstrated that the mRNA expression of sialylated voltage-dependent calcium ion channel (Cav1. 2), which is a target of influenza A virus, and the mRNA expression of angiotensin-converting enzyme 2 and transmembrane protease serine 2, which are targets of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), were significantly decreased in the EAT group (48), (49). They demonstrated also that the SARS-CoV-2 spike mRNA remaining in the epipharyngeal mucosa in the patient with long COVID disappeared by EAT along with the decrease in the mRNA expression of IL-6 and TNF-α (50). These results indicate that EAT not only suppresses inflammatory cytokine production but also has the function of eliminating the virus, making it a new treatment for long COVID.
Mogitate (51) analyzed the dynamics of CD4 cells in epipharyngeal leak fluid shed by the bloodletting effect of EAT in 18 patients with chronic epipharyngitis. As a result, it was found that the number of CD4 cells in chronic epipharyngitis was significantly higher than in healthy subjects, and that repeated EAT significantly decreased the number of CD4 cells in parallel with the recovery of symptoms. These results suggest that CD4 cells in the epipharyngeal mucosa are involved in chronic epipharyngitis and that EAT has the effect of recovering symptoms by shedding CD4 cells through its bloodletting effect.
As shown in Chapter III, vagal responses have anti-inflammatory and hypothalamus-limbic system regulation effects. Recently, VNS therapy, which utilizes such vagal responses to stimulate the afferent vagus nerve, has been used as an effective treatment for not only in refractory neurological and inflammatory diseases (52), (53), but also for long COVID (54) and myalgic encephalomyelitis (55). Initially, direct electrical stimulation of the cervical vagus nerve was performed by invasive methods, but recently, the mainstream method is to electrically stimulate the left neck or auricle, where the afferent fibers of the vagus nerve are distributed, as a method of percutaneous VNS (56), (57). Neurons can be stimulated in various ways, including light, sound, electricity, tactile stimulation, and manual massage (58). Therefore, the EAT stimulates the vagus nerve afferent fibers abundant in the epipharynx. The STN, located in the medulla oblongata just a few centimeters behind the epipharynx, is stimulated, which results in the activation of the vagal response. In this way, various functional somatic symptoms are also improved. Ito (27), (28), (29), (30), (59) has demonstrated that EAT has a VNS effect by multidimensional autonomic nerve function test.
The following are the reports of EAT effects that have been published to date.
Mogitate (60) reported that after 6 to 9 months of repeated EAT in 74 patients, endoscopic findings disappeared in 86% of patients and subjective symptoms disappeared in 70% of patients. Ohno (61) evaluated 154 patients who underwent EAT. He showed that the improvement rate of the endoscopic findings was 76%, and the improvement rate of the chief complaint was 86%, indicating a significant correlation between the improvement of the local findings and the improvement of the chief complaint.
Ohno (62) reported that in 110 patients who complained of headaches underwent EAT once or twice a week for a total of 10 visits, 83 patients (76%) showed improvement. Kusuyama (63) reported that 53 patients with chronic epipharyngitis complaining of voice disturbances were treated with EAT 10-20 times, and significant improvement was observed in endoscopic findings, subjective evaluation, and longest vocal duration. Kitajima (64) studied the effect of EAT on 168 diver patients (83 with EAT and 85 without EAT) with ear pressure trauma associated with Eustachian tube dysfunction and showed significant improvement in the opening pressure in the EAT group.
Shin (25) performed EAT on 34 patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and evaluated its effect on the symptom severity index. The results showed that recurrent flu-like symptoms decreased from 0.38 to 0.11, memory impairment from 0.47 to 0.17, and total score of severity from 26.7 to 9.3 all showed significant improvement. Ito (30) reported that when EAT was administered to ME/CFS patients with chronic epipharyngitis, salivary α-amylase activity and salivary cortisol secretion, which are indicators of the hypothalamus-pituitary-adrenal cortex axis, and heart rate variability in the orthostatic stress test, which is an indicator of autonomic nervous function, improved, and that EAT normalizes parasympathetic and sympathetic reflexes over time and adjusts the autonomic nervous balance.
Hotta (26), (44) found that in 41 young women after HPV vaccination, their symptoms were headache 97%, general fatigue 95%, sleep disturbance 88%, stiff shoulders 85%, menstrual disorders 78%, dizziness 76%, muscle weakness 76%, tinnitus 68%, abdominal pain/diarrhea 65%, generalized pain 61%, arthralgia 58%, fever 48%, and 82% were unable to attend school. When EAT was performed in 16 of these patients, 13 (81%) showed improvement in symptoms, and 4 (25%) were eventually cured.
Imai et al. (8) reported that all 58 long COVID patients had epipharyngeal inflammation as seen by endoscopy. They performed EAT once a week for 1 month and evaluated the degree of improvement in symptoms using a visual analog scale (VAS). As a result, they found that all symptoms improved significantly: general fatigue decreased from 65 to 23, headache from 49 to 11, and impaired concentration from 52 to 36. Saito and Komori (65) performed EAT, 12 times, once every 1-2 weeks, in 49 long COVID patients, and evaluated 10 symptoms on a 0-10 scale. As a result, they found that all symptom scores significantly improved at the time of the final visit, and 39 (80%) patients showed over 70% symptom improvement rate.
Hotta (36), (37), the developer of tonsillectomy plus steroid pulse (TSP) therapy reported that 682 (99%) of 686 patients with IgA nephropathy had epipharyngitis findings. He (37) found that in 24 patients with refractory IgA nephropathy (19 TSP-resistant, 5 relapse after remission with TSP) who were treated with EAT for 1-26 months, 20 patients (83%) showed resolution of hematuria. Furthermore, he (36) found that in 49 patients with latent IgA nephropathy with urinary occult blood but no urinary protein, hematuria disappeared in 28 (67%) of 42 patients with EAT, while urinary occult blood did not disappear in 7 patients without EAT. Mogitate (66) reported significant improvement in hematuria and proteinuria after EAT in 21 patients with IgA nephropathy resisting TSP. Doi et al. (67) performed EAT 40 times in 4 patients with IgA nephropathy that recurred or relapsed after TSP, and found that all patients showed improvement of urinary occult blood. Kaneko et al. (68) reported that EAT combined with steroid pulse therapy was effective in a 65-year-old man with IgA nephropathy and psoriatic arthritis. Fujimoto et al. (69) reported a patient with recurrent IgA vasculitis after renal transplantation who responded to steroid pulse therapy and daily EAT for 3 months. With regard to palmoplantar pustulosis, Imai (70) reported that 30 (81%) of 37 patients with EAT for more than 3 months showed improvement in their skin eruption.
The EAT Review Committee has been conducting a multicenter prospective study on the efficacy of EAT for chronic epipharyngitis since November 2021 (research theme: “Prospective Study on the Efficacy of Epipharyngeal Abrasion Therapy,” Ethics Review Committee of Asahikawa Medical University, Approval Number 21177). The subjects were patients who met the diagnostic criteria for chronic epipharyngitis (Chapter II) and were 16 years or older. The epipharyngeal score was defined as the sum of the 4 endoscopic finding scores for epipharyngitis (Figure 1). A visual analog scale (VAS) was used for the symptom score. EAT was administered once a week for 8 weeks (8 times in total).
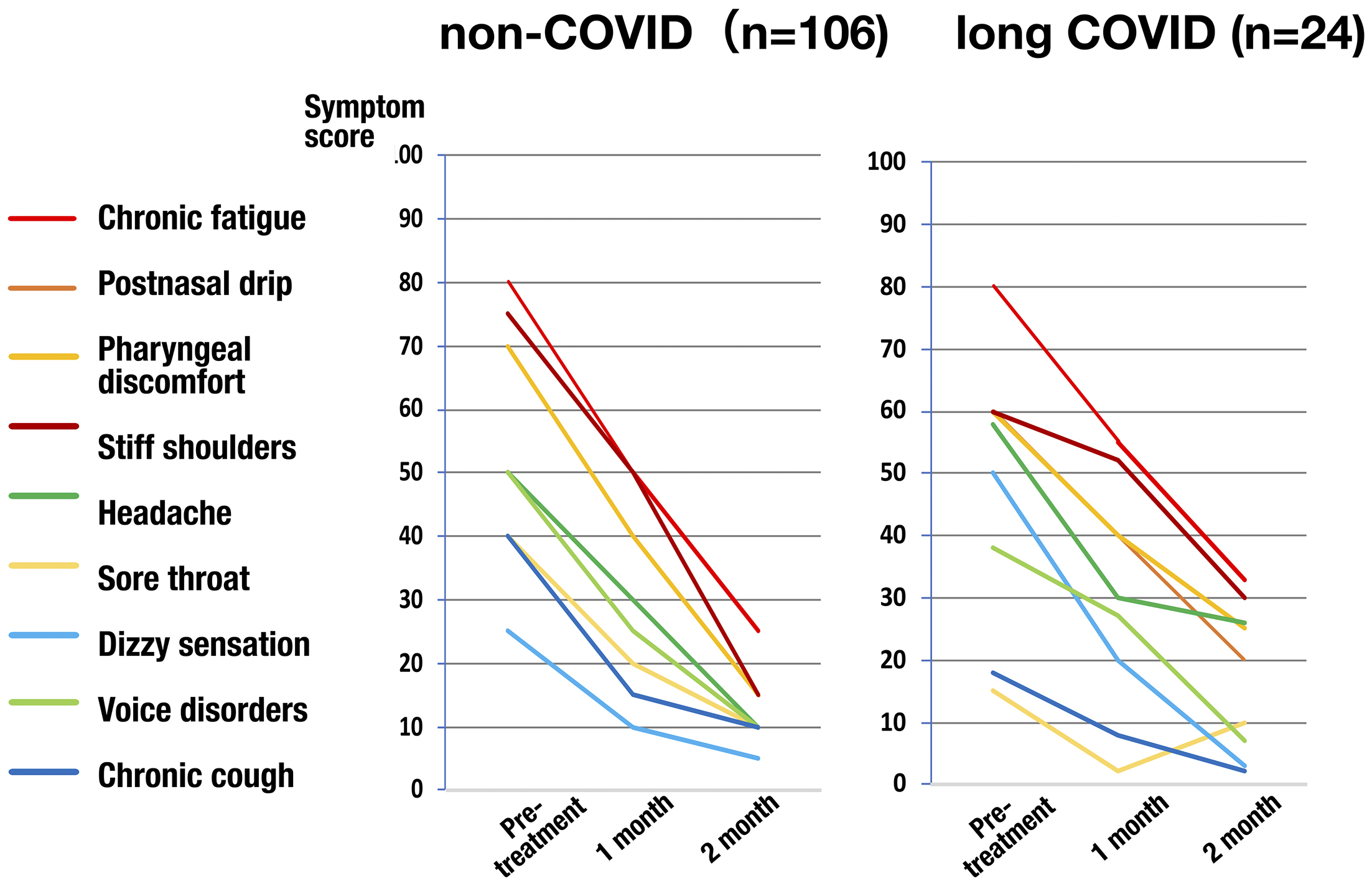
The interim analysis subjecting to 130 patients including 106 non-COVID patients and 24 long COVID patients, 32 men and 98 women, aged 15 to 84 years (median 44 years), who were enrolled from the start of the survey until March 2023, was performed. Both groups of non-COVID and long COVID had various symptoms, but the majority of chief complaints were postnasal drip (30%) and pharyngeal discomfort (18%) in non-COVID patients, and chronic fatigue (42%) in long COVID patients. Every symptom score significantly decreased over time (Figure 8). The epipharyngeal score also significantly decreased. The symptom improvement rate of the chief complaint 2 months after the start of EAT was calculated, and 42% of patients showed significant improvement (symptom improvement rate: ≥80%), 27% showed improvement (symptom improvement rate: 50%-80%), and 11% showed slight improvement (symptom improvement rate: 30%-50%). Therefore, 80% of patients experienced “more than slight improvement,” providing objective evidence that EAT is a highly effective treatment for chronic epipharyngitis with both non-COVID and long COVID.
With the recent development of neuroimmunology, which links the nervous and immune systems, the mechanisms of vagal responses in the epipharynx, cerebrospinal fluid outflow pathways, and the dynamics of cytokines and viral particles have been clarified, and then, the pathogenesis of chronic epipharyngitis and the mechanism of EAT effects has been elucidated. Chronic epipharyngitis is a common disease with a wide variety of symptoms and a considerable number of patients. Recently, the effectiveness of EAT for long COVID has been highlighted, and it is expected that the number of patients eligible for EAT will increase. The majority of patients who complain of only functional somatic symptoms such as fatigue, headache, dizziness, palpitations, abdominal pain, diarrhea, insomnia, etc., without otolaryngological symptoms, may initially visit a general physician. However, it may be difficult for general physicians to definitively diagnose chronic epipharyngitis by performing epipharyngeal endoscopy. If no organic disease is detected in such patients through gastrointestinal endoscopy, imaging tests, and physiological tests, it would be better to consult an otolaryngologist with this disease in mind. Alternatively, it may be good also to treat such patients with pharmacotherapy as a functional somatic disease such as long COVID, ME/CFS, etc., and if there is little improvement, refer them to an otolaryngologist. Therefore, in the treatment of chronic epipharyngitis, it is important to establish a treatment system consisting of close collaboration between general physicians and otolaryngologists, and for this purpose, not only otolaryngologists but also general physicians need to deepen their understanding and knowledge of chronic epipharyngitis and explain it to patients. Such a strategy should alleviate the suffering of many distressed patients.
None
All authors listed in the manuscript meet the following 4 contribution criteria: (1) Substantial contributions to the conception or design of the research or the acquisition and analysis of data; (2) Drafting the work or reviewing it critically for important intellectual content; (3) Final approval of the version to be published; (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Yamazaki S. [Nasopharyngitis]. Jibiinkoka. 1970;42(10):807-13. Japanese.
Yamazaki S. [Nasopharyngitis syndrome, symptom and pathological study]. Otolaryngology (Tokyo). 1961;33:97-101. Japanese.
Horiguchi S. [Epipharyngitis and its relationship to general diseases]. Nihon Jibiinkoka Gakkai Kaiho. 1966;69:1-82. Japanese.
Horiguti S. Nasopharyngitis. Acta Otolaryngol Suppl. 1975;329:1-120.
Horiguchi S. The discovery of the nasopharyngitis and its influence on general diseases. Acta Otolaryngol. 1975;329(suppl):1-120.
Hotta O, Inoue CN, Tanaka A, et al. Possible mechanisms underlying epipharyngeal abrasive therapy (EAT) with ZnCl2 solution for the treatment of autoimmune diseases and functional somatic syndrome. J Antivir Antiretrovir. 2017;9(4):81-6.
Tanaka A. [Studies on band-limited light endoscopic diagnosis and enscopic epipharyngeal abrasive therapy in chronic epipharyngitis]. Stomato-Pharyngology. 2018;31:57-67. Japanese.
Imai K, Yamano T, Nishi S, et al. Epipharyngeal abrasive therapy (EAT) has potential as a novel method for long COVID treatment. Viruses. 2022;14(5):907.
Adam D. How long is COVID infectious? What scientists know so far. Nature. 2022;608(7921):16-7.
Medical frontiers. Japanese treatment EAT for long COVID [Internet]. NHK World Japan; 2023 [cited 2023 May 23]. Available from: https://www3.nhk.or.jp/nhkworld/en/shows/2050141/
Horiguti S, Ide Y. Study on epipharyngitis upon the basis of exfoliative cytological observation of epipharyngeal mucosa. Bull Tokyo Med Dent Univ. 1963;10:441-58.
Horiguti S, Tanaka S. A study on the correlation between the clinical course of epipharyngitis and the activity of fibrinolytic system. Bull Tokyo Med Dent Univ. 1966;13(4):467-88.
Horiguchi S, Murakami A. A morphological study of ciliated epithelial cells in epipharyngitis. Bull Tokyo Med Dent Univ. 1967;14(2):157-72.
Harabuchi Y, Hamamoto M, Kodama H, et al. Spontaneous immunoglobulin production by adenoidal and tonsillar lymphocytes in relation to age and otitis media with effusion. Int J Pediatr Otorhinolaryngol. 1996;35(2):117-25.
Jara LJ, Izquierdo E, Medina G. Is the immune neuroendocrine system the connection between epipharyngitis and chronic fatigue syndrome induced by HPV vaccine?: editorial. Immunol Res. 2017;65(1):5-7.
Sluder G. The syndrome of sphenopalatine ganglion neurosis. Am J Med Sci. 1910;140(6):868.
Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. 2016;594(20):5781-90.
Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853-9.
EXPRESSION OF CONCERN NOTICE to “Darwin revisited: the vagus nerve is a causal element in controlling recognition of other’s emotions” by Lorenza S. Colzato, Roberta Sellaro, Christian Beste [Cortex. 2017;92:95-102]. Cortex. 2022;152:165.
Nail BS, Sterling GM, Widdicombe JG. Epipharyngeal receptors responding to mechanical stimulation. J Physiol. 1969;204(1):91-8.
Gandevia SC, McCloskey DI, Potter EK. Reflex bradycardia occurring in response to diving, nasopharyngeal stimulation and ocular pressure, and its modification by respiration and swallowing. J Physiol. 1978;276:383-94.
Miyaoka Y, Takahashi Y, Sato S, et al. Autonomic nervous reflexes in respiration elicited by mechanical stimulation of the velopharyngeal region in rabbits. J Auton Nerv Syst. 1989;26(2):177-80.
Colzato LS, Elmers J, Beste C, et al. A prospect to ameliorate affective symptoms and to enhance cognition in long COVID using auricular transcutaneous vagus nerve stimulation. J Clin Med. 2023;12(3):1198.
Martins DF, Viseux FJF, Salm DC, et al. The role of the vagus nerve in fibromyalgia syndrome. Neurosci Biobehav Rev. 2021;131:1136-49.
Shin I, Hotta O, Tani S. [Epipharyngeal abrasive therapy in myalgic encephalomyelitis/chronic fatigue syndrome patients]. Nippon Rinsyo. 2021;79:989-94. Japanese.
Hotta O, Ieiri N, Inoue NC, et al. Chronic epipharyngitis: a missing trigger in chronic fatigue syndrome. J Transl Sci. 2018;4:1-9.
Hirobumi I. Autonomic nervous system regulation effects of epipharyngeal abrasive therapy for myalgic encephalomyelitis/chronic fatigue syndrome associated with chronic epipharyngitis. Cureus. 2023;15(1):e33777.
Hirobumi I. The effect of epipharyngeal abrasive therapy (EAT) on the baroreceptor reflex (BR). Cureus. 2023;15(9):e45080.
Hirobumi I. Autonomic stimulation action of EAT (epipharyngeal abrasive therapy) on chronic epipharyngitis. Cureus. 2024;16(6):e63182.
Hirobumi I. A case report of chronic epipharyngitis with chronic fatigue treated with epipharyngeal abrasive therapy (EAT). Cureus. 2024;16(2):e54742.
Hulens M, Dankaerts W, Rasschaert R, et al. The link between empty Sella syndrome, fibromyalgia, and chronic fatigue syndrome: the role of increased cerebrospinal fluid pressure. J Pain Res. 2023;16:205-19.
Wostyn P. COVID-19 and chronic fatigue syndrome: is the worst yet to come? Med Hypotheses. 2021;146:110469.
Yoon JH, Jin H, Kim HJ, et al. Nasopharyngeal lymphatic plexus is a hub for cerebrospinal fluid drainage. Nature. 2024;625(7996):768-77.
Harabuchi Y, Takahara M. Pathogenic role of palatine tonsils in palmoplantar pustulosis: a review. J Dermatol. 2019;46(11):931-9.
Harabuchi Y, Takahara M. Recent advances in the immunological understanding of association between tonsil and immunoglobulin A nephropathy as a tonsil-induced autoimmune/inflammatory syndrome. Immun Inflamm Dis. 2019;7(2):86-93.
Hotta O, Ieiri N, Nagai M, et al. Role of palatine Tonsil and epipharyngeal Lymphoid Tissue in the Development of Glomerular Active Lesions (Glomerular vasculitis) in immunoglobulin A Nephropathy. Int J Mol Sci. 2022;23(2):727.
Hotta O, Tanaka A, Oda T. Chronic epipharyngitis: a missing background of IgA nephropathy. Autoimmun Rev. 2019;18(8):835-6.
Hotta O, Oda T. The epipharynx-kidney axis triggers glomerular vasculitis in immunoglobulin A nephropathy. Immunol Res. 2019;67(4-5):304-9.
Harabuchi Y. Techniques for E-EAT. Vols. 1-1. Transnasal Method (overview; presented by Dr. Tanaka, A.) [Internet]. Youtube Studio; 2024 [cited 2024 Oct 20]. Available from: https://youtu.be/nTV3I4ZgITw
Harabuchi Y. Techniques for E-EAT. Vols. 1-2. Transnasal Method (endoscopic view; presented by Dr. Tanaka, A.) [Internet]. Youtube Studio; 2024 [cited 2024 Oct 20]. Available from: https://youtu.be/Go5FkOYjOO0
Harabuchi Y. Techniques for E-EAT. Vols. 1-3. Strongly-Curved Cotton Applicator Method (presented by Dr. Tanaka, A.) [Internet]. Youtube Studio; 2024 [cited 2024 Oct 20]. Available from: https://youtu.be/tF9AM4c7Ecc
Harabuchi Y. Techniques for E-EAT. Vols. 2-1. Transoral Method (overview; presented by Dr. Tanaka, A.) [Internet]. Youtube Studio; 2024 [cited 2024 Oct 20]. Available from: https://youtu.be/l4PaEg_TqB4
Harabuchi Y. Techniques for E-EAT. Vols. 2-2. Transoral Method (endoscopic view; presented by Dr. Tanaka, A.) [Internet]. Youtube Studio; 2024 [cited 2024 Oct 20]. Available from https://youtu.be/peP0bcszlcY
Hotta O, Tanaka A, Torigoe A, et al. Involvement of chronic epipharyngitis in autoimmune (auto-inflammatory) syndrome induced by adjuvants (Asia). Immunol Res. 2017;65(1):66-71.
Mogitate M. Effectiveness of epipharyngeal abrasive therapy on chronic epipharyngitis and the exhaled nitric oxide levels. Intern Med. 2023;62(8):1139-44.
Mogitate M. Exhaled nitric oxide levels are associated with the severity of chronic epipharyngitis and decreased via epipharyngeal abrasion. Auris Nasus Larynx. 2023;50(4):534-9.
Nishi K, Yoshimoto S, Nishi S, et al. Epipharyngeal abrasive therapy (EAT) reduces the mRNA expression of major proinflammatory cytokine IL-6 in chronic epipharyngitis. Int J Mol Sci. 2022;23(16):9205.
Nishi K, Yoshimoto S, Nishi S, et al. Epipharyngeal abrasive therapy down-regulates the expression of Cav1.2: a key molecule in influenza virus entry. In Vivo. 2022;36(5):2357-64.
Nishi K, Yoshimoto S, Nishi S, et al. Epipharyngeal abrasive therapy down-regulates the expression of SARS-CoV-2 entry factors ACE2 and TMPRSS2. In Vivo. 2022;36(1):371-4.
Nishi K, Yoshimoto S, Tanaka T, et al. A potential novel treatment for chronic cough in long COVID patients: clearance of epipharyngeal residual SARS-CoV-2 spike RNA by epipharyngeal abrasive therapy. Cureus. 2023;15(1):e33421.
Mogitate M. Epipharynegal abrasive therapy downregulates the number of epipharyngeal abrasive CD4 cells with symptomatic recovery. Cureus. 2023;15(12):e50288.
Koopman FA, Chavan SS, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113(29):8284-9.
Bonaz B, Sinniger V, Hoffmann D, et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 2016;28(6):948-53.
Badran BW, Huffman SM, Dancy M, et al. A pilot randomized controlled trial of supervised, at-home, self-administered transcutaneous auricular vagus nerve stimulation (taVNS) to manage long COVID symptoms. Bioelectron Med. 2022;8(1):13.
Rodriguez L, Pou C, Lakshmikanth T, et al. Achieving symptom relief in patients with myalgic encephalomyelitis by targeting the neuro-immune interface and optimizing disease tolerance. Oxf Open Immunol. 2023;4(1):iqad003.
Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part II. Headache. 2016;56(2):259-66.
Zhao YX, He W, Jing XH, et al. Transcutaneous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysaccharide-induced inflammation. Evid Based Complement Alternat Med. 2012;2012:627023.
Mahmoudi P, Veladi H, Pakdel FG. Optogenetics, tools and applications in neurobiology. J Med Signals Sens. 2017;7(2):71-9.
Hirobumi I. A successful case report of epipharyngeal abrasive therapy (EAT) in a patient with sleep apnea syndrome and traumatic tympanic membrane perforation caused by chronic epipharyngitis. Cureus. 2024;16(4):e59089.
Mogitate M, Sasaki Y, Komiyama A. Outcome of an outpatient specialty clinic for chronic epipharyngitis. Auris Nasus Larynx. 2021;48(3):451-6.
Ohno Y. [Headache, dizziness]. Nippon Rinsyo. 2021;79:995-9. Japanese.
Ohno Y. Use of nasopharyngoscopy severity classification of chronic epipharyngitis and its application for evaluating the treatment outcomes of epipharyngeal abrasive therapy. Cureus. 2024;16(2):e54067.
Kusuyama T, Nakagawa H. [A clinical study of cases with voice disorder caused by nasopharyngitis in singers]. Stomato-Pharyngology. 2020;33:59-63. Japanese.
Kitajima N. [Efficacy of epipharyngeal abrasive therapy for Eustachian tube dysfunction in scuba divers with ear barotrauma]. Pract Oto Rhinolaryngol. 2024;117(6):499-505. Japanese.
Saito Y, Komori M. [Efficacy of epipharyngeal abrasibe therapy for post-COVID-19]. J Immunol Allergy Infect Orl. 2022;2:153-9. Japanese.
Mogitate M. Epipharyngeal abrasive therapy for patients with immunoglobulin A nephropathy: a retrospective study. SJO. 2022;8(3):874-81.
Doi A, Kozakura K, Dehara Y, et al. [Epipharyngeal abrasive therapy in patients with recurrent/relapse IgA nephropathy after tonsillectomy]. Stomato-Pharyngology. 2024;37:128-33. Japanese.
Kaneko T, Mii A, Fukui M, et al. IgA nephropathy and psoriatic arthritis that improved with steroid pulse therapy and mizoribine in combination with treatment for chronic tonsillitis and epipharyngitis. Intern Med. 2015;54(9):1085-90.
Fujimoto M, Katayama K, Nishikawa K, et al. A kidney transplant recipient with recurrent Henoch-Schönlein purpura nephritis successfully treated with steroid pulse therapy and epipharyngeal abrasive therapy. Nephron. 2020;144(suppl 1):54-8.
Imai I. [Epipharyngeal abrasive therapy for palmoplantar pustulosis and similar diseases]. Nippon Rinsyo. 2021;79:1054-9. Japanese.