Corresponding author: Takashi Matsukawa, matsukawa1012@g.ecc.u-tokyo.ac.jp
DOI: 10.31662/jmaj.2025-0067
Received: February 8, 2025/
Accepted: April 23, 2025
Advance Publication: June 27, 2025
Key words: neurosarcoidosis, nonfluent aphasia, splenomegaly, splenic biopsy, epithelioid cell granuloma
A 19-year-old right-handed previously healthy male presented with episodic, nonfluent aphasia lasting approximately 1 hour every few days for 4 months. Brain magnetic resonance imaging (MRI) revealed multiple hyperintense lesions in the superior temporal gyrus and supramarginal gyrus in fluid-attenuated inversion recovery images with linear contrast enhancement along the medullary veins (Figure 1A and B). Dilated medullary veins and multiple microbleeds were also noted in susceptibility-weighted imaging (Figure 1C). Abdominal MRI revealed mild splenomegaly and a heterogeneously enhanced lesion with variable contrast enhancement during the portal venous phase (Figure 1D). 18F-fluorodeoxyglucose positron emission tomography/computed tomography showed abnormal uptake corresponding to this splenic region, with a maximal standardized uptake value of 3.1 (Figure 1E). Ultrasound-guided splenic biopsy confirmed clustered epithelioid cell granulomas without caseous necrosis (Figure 1F), leading to the diagnosis of probable neurosarcoidosis. Comprehensive evaluations revealed no evidence of abnormalities in any other organs, including those in the pulmonary, cutaneous, cardiac, or ophthalmologic system.
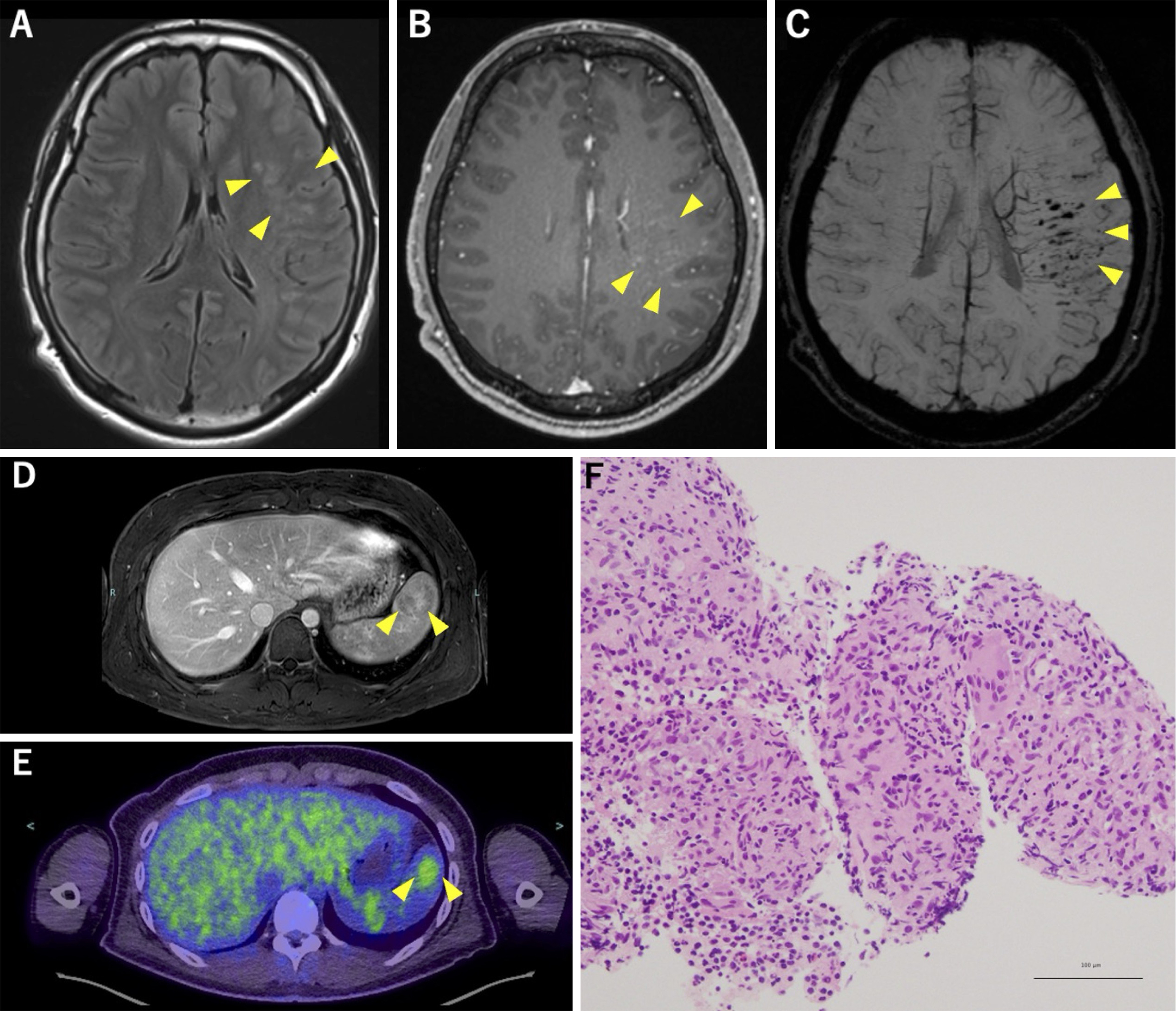
Seizures in neurosarcoidosis (1) may result from brain parenchymal, meningeal lesions, or granuloma-associated small vessel vasculitis (2). In sarcoidosis, cases without pulmonary involvement are uncommon (8%) (3), and reports of lesions confined solely to the central nervous system (CNS) and spleen are exceedingly rare. However, considering the challenges associated with obtaining CNS biopsies, it is noteworthy that splenic biopsy aids in diagnosis.
None
Satoka Yano and Takashi Matsukawa acquired data and drafted the manuscript. Kensho Sumi, Reo Yoshioka, Yusuke Baba, Hirotaka Maekawa, Masashi Hamada, Wataru Satake, Masako Ikemura, and Tatsushi Toda edited and approved the final manuscript.
Informed consent was obtained from the patient.
IRB approval was not required for this study.
Fritz D, van de Beek D, Brouwer MC. Clinical features, treatment and outcome in neurosarcoidosis: systematic review and meta-analysis. BMC Neurol. 2016;16(1):220.
Lacomis D. Neurosarcoidosis. Curr Neuropharmacol. 2011;9(3):429-36.
James WE, Koutroumpakis E, Saha B, et al. Clinical features of extrapulmonary sarcoidosis without lung involvement. Chest. . 2018;154(2):349-56.