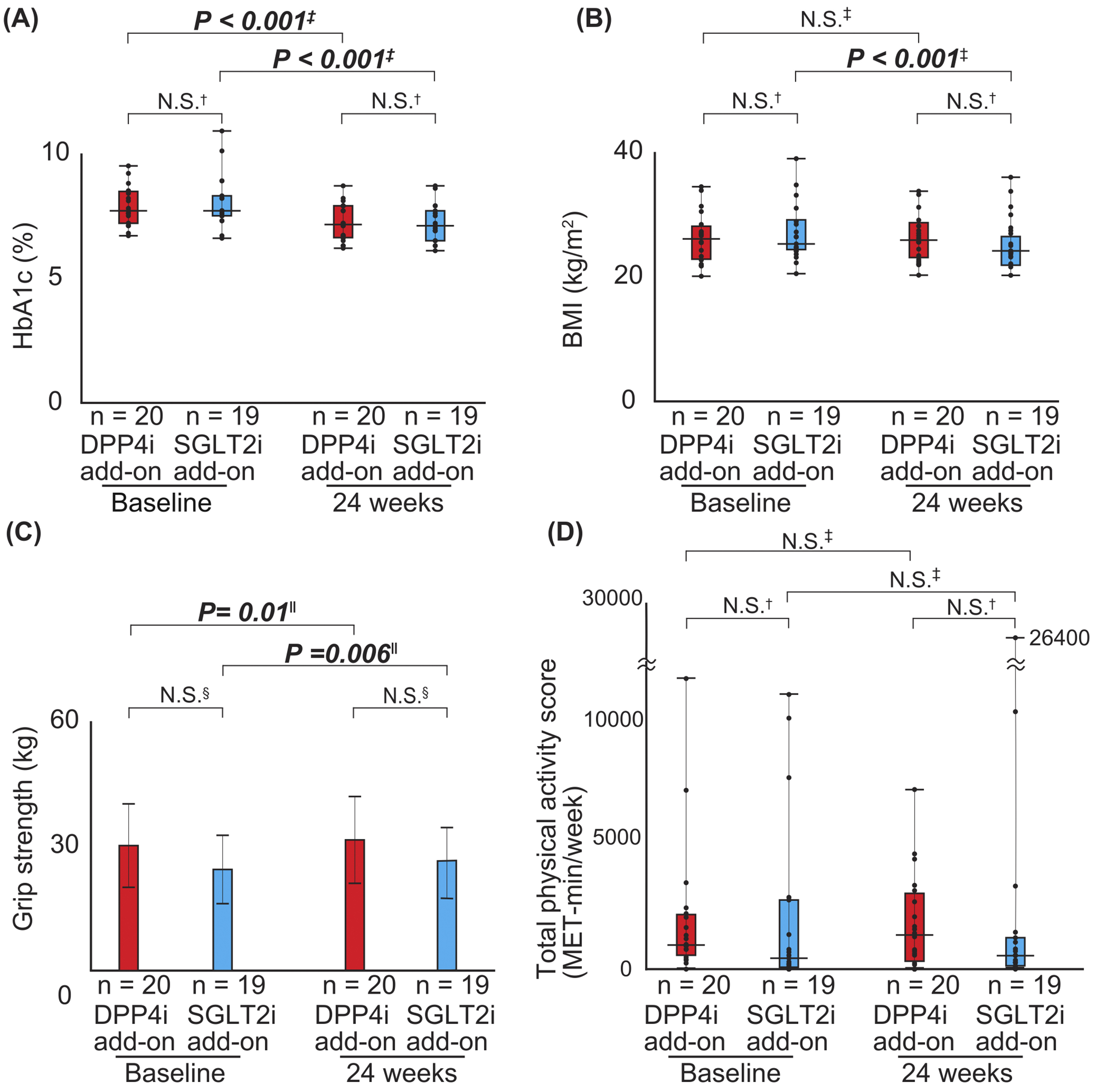
Figure 1. Study flowchart and definition of the evaluated parameters
(A) Study flowchart: Patients with type-2 diabetes mellitus who had been treated with linagliptin or empagliflozin for ≥3 months were included in this study. The clinical data at both baseline and 24 weeks were evaluated. In addition, the patients were separated into two subgroups based on the preceding medications: the DPP4i and SGLT2i add-on groups. The clinical parameters were evaluated before (baseline) and after (24 weeks) combination therapy. (B) Timeline of the study protocol. (C) Definitions of parameters evaluated in this study are listed.
BMI, body mass index; DPP4i, dipeptidyl peptidase-4 inhibitor; GDS5, Geriatric Depression Scale 5; IPAQ-SF, International Physical Activity Questionnaire Short Form; QOL, quality of life; SGLT2i, sodium-glucose cotransporter-2 inhibitor.
From: Assessing the Metabolic and Physical Effects of Combined DPP4 and SGLT2 Inhibitor Therapy in Patients with Type-2 Diabetes Mellitus: An Observational Prospective Pilot Study
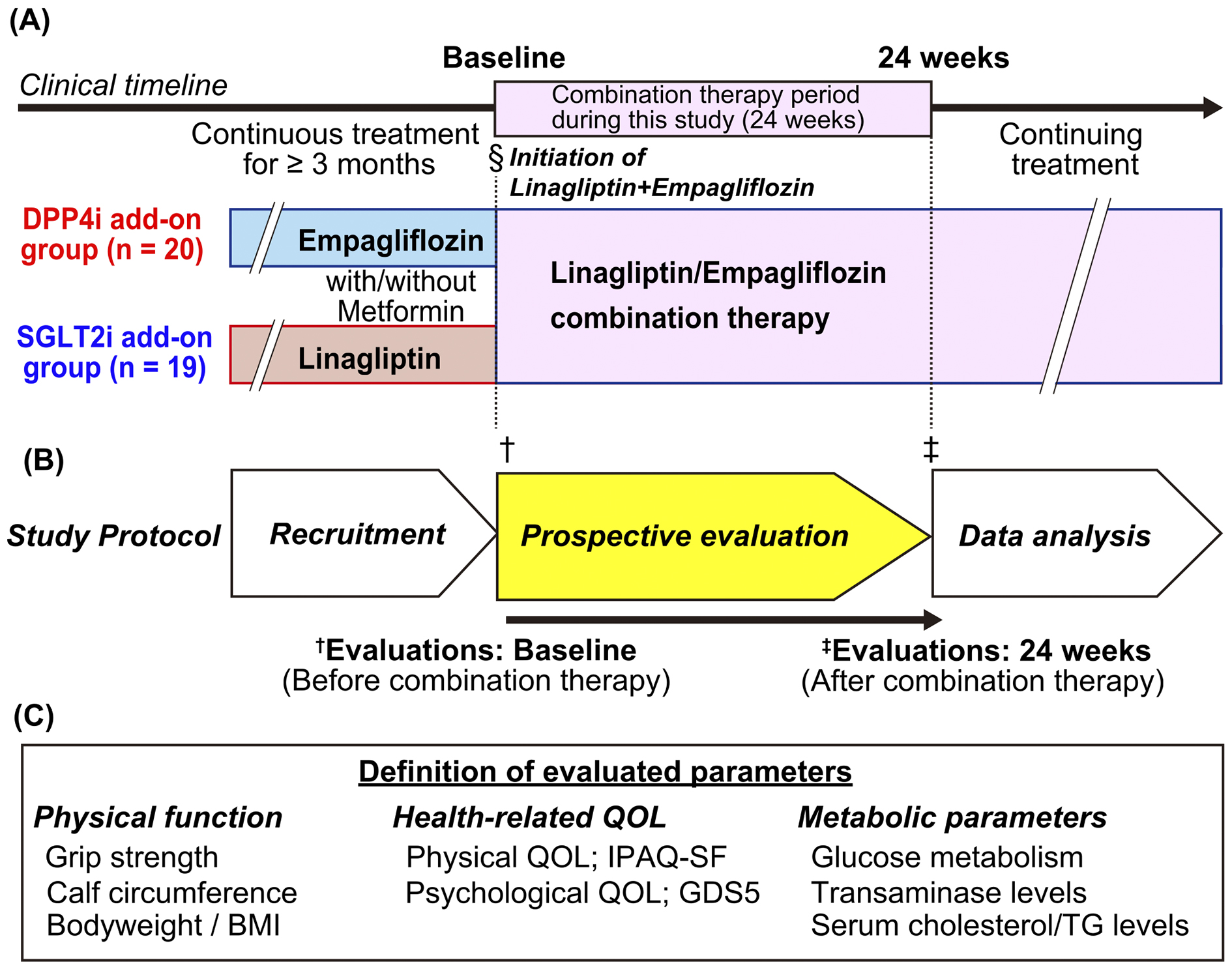
Figure 3. Changes in physical functions and QOL in the DPP4i (n = 20) and SGLT2i (n = 19) add-on groups
Changes in the body mass index, grip strength, calf circumference, and total physical activity score of the SGLT2i and DPP4i add-on groups are presented. Each value is presented as the mean (red box, DPP4i add-on group; blue box, SGLT2i add-on group) and standard deviation (bars) for BMI, grip strength, and calf circumference or median (horizontal lines in colored boxes), interquartile range (IQR) (colored boxes), and range (bars) for total physical activity score. (A) Body mass index (BMI). (B) Grip strength. (C) Calf circumference, left. (D) Calf circumference, right. (E) Total physical activity score. Analyses were performed using Student’s t-test (†) or Mann-Whitney U test (‡). *P < 0.05, **P < 0.01.
DPP4i, dipeptidyl peptidase-4 inhibitor; SGLT2i, sodium-glucose cotransporter-2 inhibitor.
From: Assessing the Metabolic and Physical Effects of Combined DPP4 and SGLT2 Inhibitor Therapy in Patients with Type-2 Diabetes Mellitus: An Observational Prospective Pilot Study
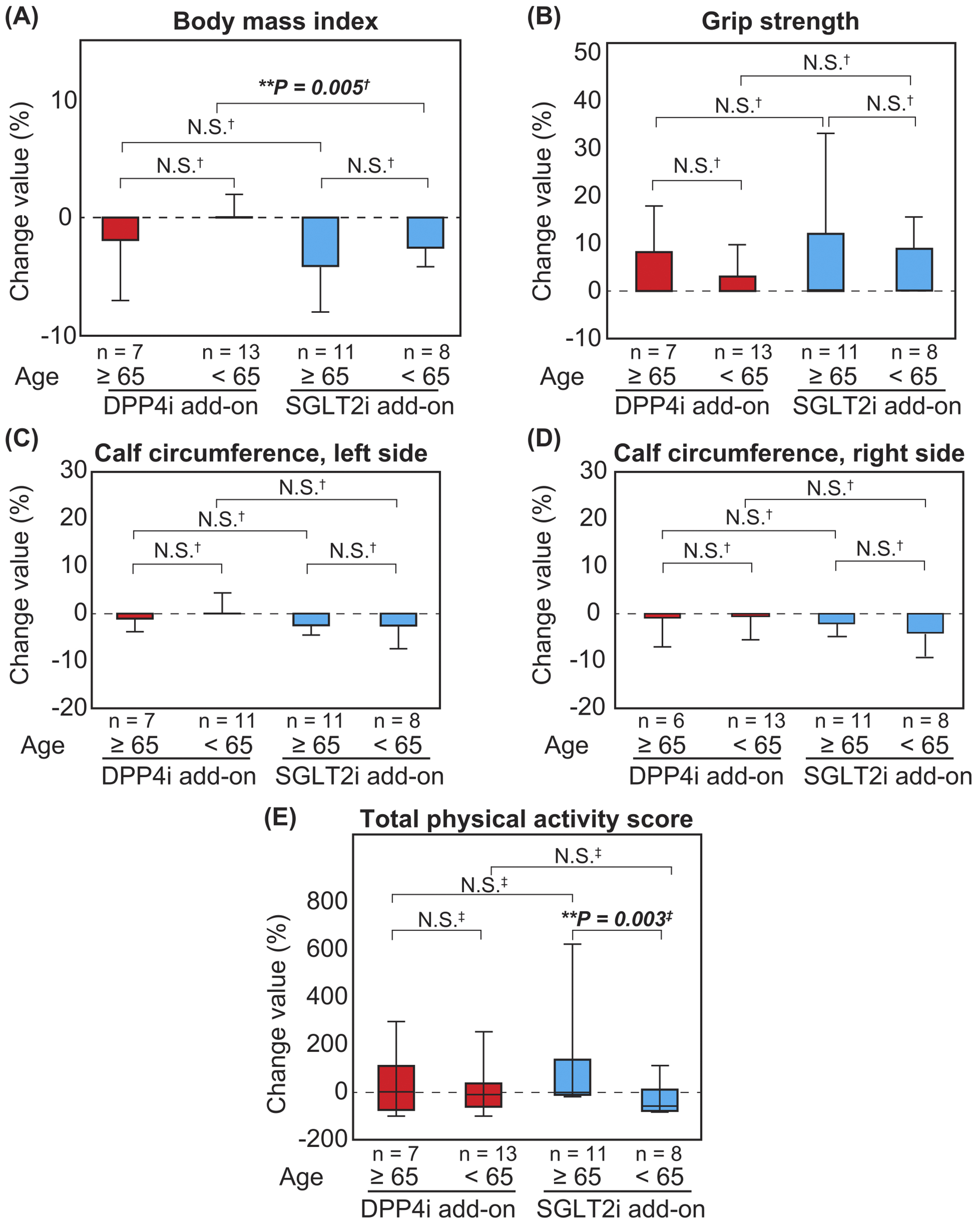
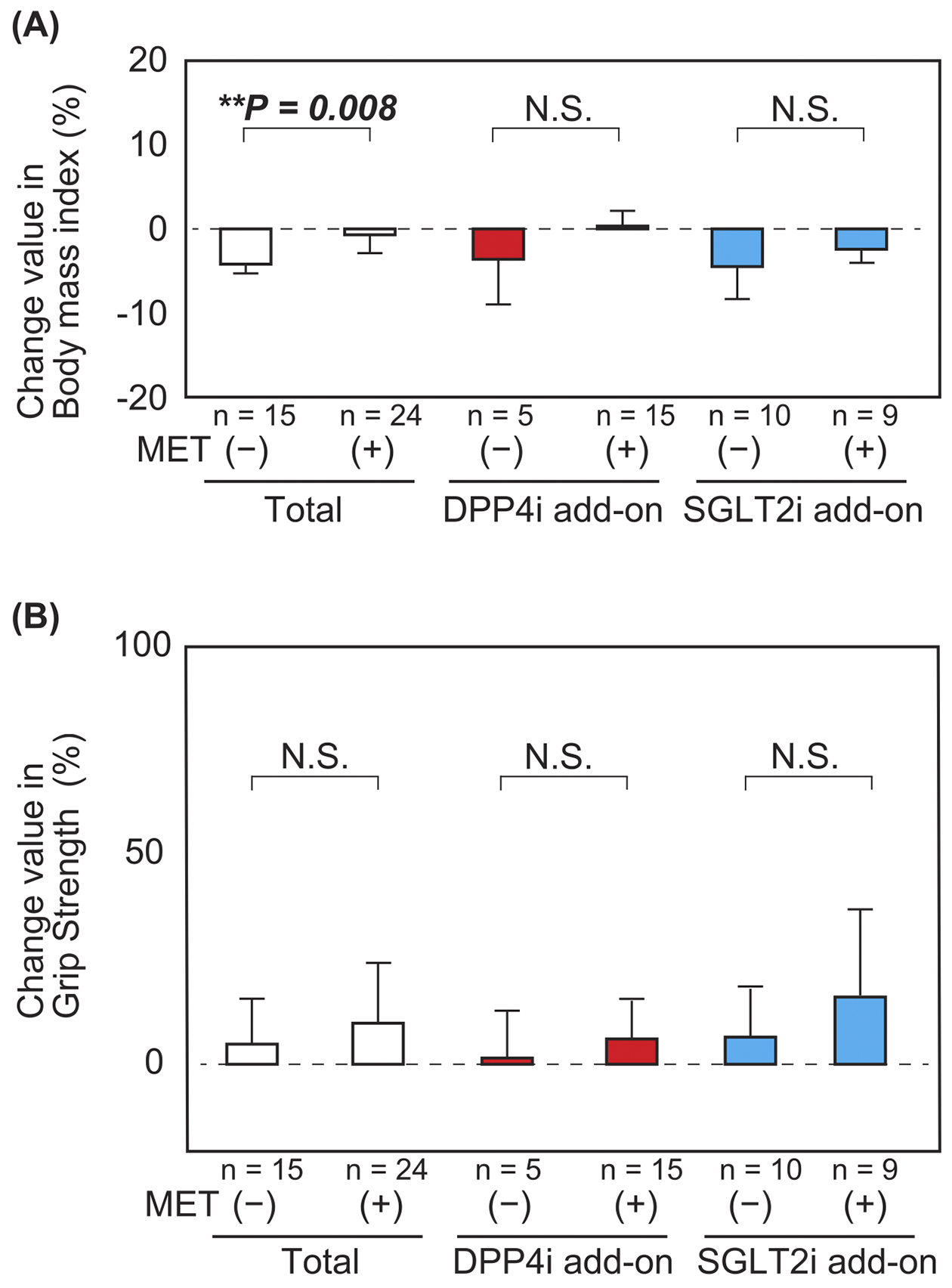
Figure 4. Changes in body weight and grip strength between before and after combination therapy with (n = 24) or without (n = 15) metformin preadministration
(A) Stratified analysis based on DPP4i or SGLT2i add-on group showed significant differences between the groups with or without metformin preuse. Body weight significantly decreased after combination therapy with DPP4i and SGLT2i in the absence of metformin. (B) There was no significant difference in grip strength between the groups with or without metformin preuse. Values are expressed as mean (open box, total group; red box, DPP4i add-on group; blue box, SGLT2i add-on group) ± SD (bars). The total body mass index (without metformin: −4.19% ± 1.10% vs. with metformin: −0.70% ± 2.18%, P = 0.008). The DPP4i add-on group (without metformin: −3.61% ± 5.37% vs. with metformin: 0.34% ± 1.82%, P = 0.18). The SGLT2i add-on group (without metformin: −4.48% ± 3.86% vs. with metformin: −2.42% ± 1.61%, P = 0.15). Analyses were performed using Student’s t-test. *P < 0.05, **P < 0.01.
DPP4i, dipeptidyl peptidase-4 inhibitor; SGLT2i, sodium-glucose cotransporter-2 inhibitor.
From: Assessing the Metabolic and Physical Effects of Combined DPP4 and SGLT2 Inhibitor Therapy in Patients with Type-2 Diabetes Mellitus: An Observational Prospective Pilot Study