Figure 1. iPSCs describe cells that have been reprogrammed to the pluripotent state. In the illustration, a somatic cell has had OSKM exogenously expressed to initiate the reprogramming mechanism. The result is an iPSC, which in proper culture conditions can be induced to differentiate into any cell type.
From: Bringing Induced Pluripotent Stem Cell Technology to the Bedside
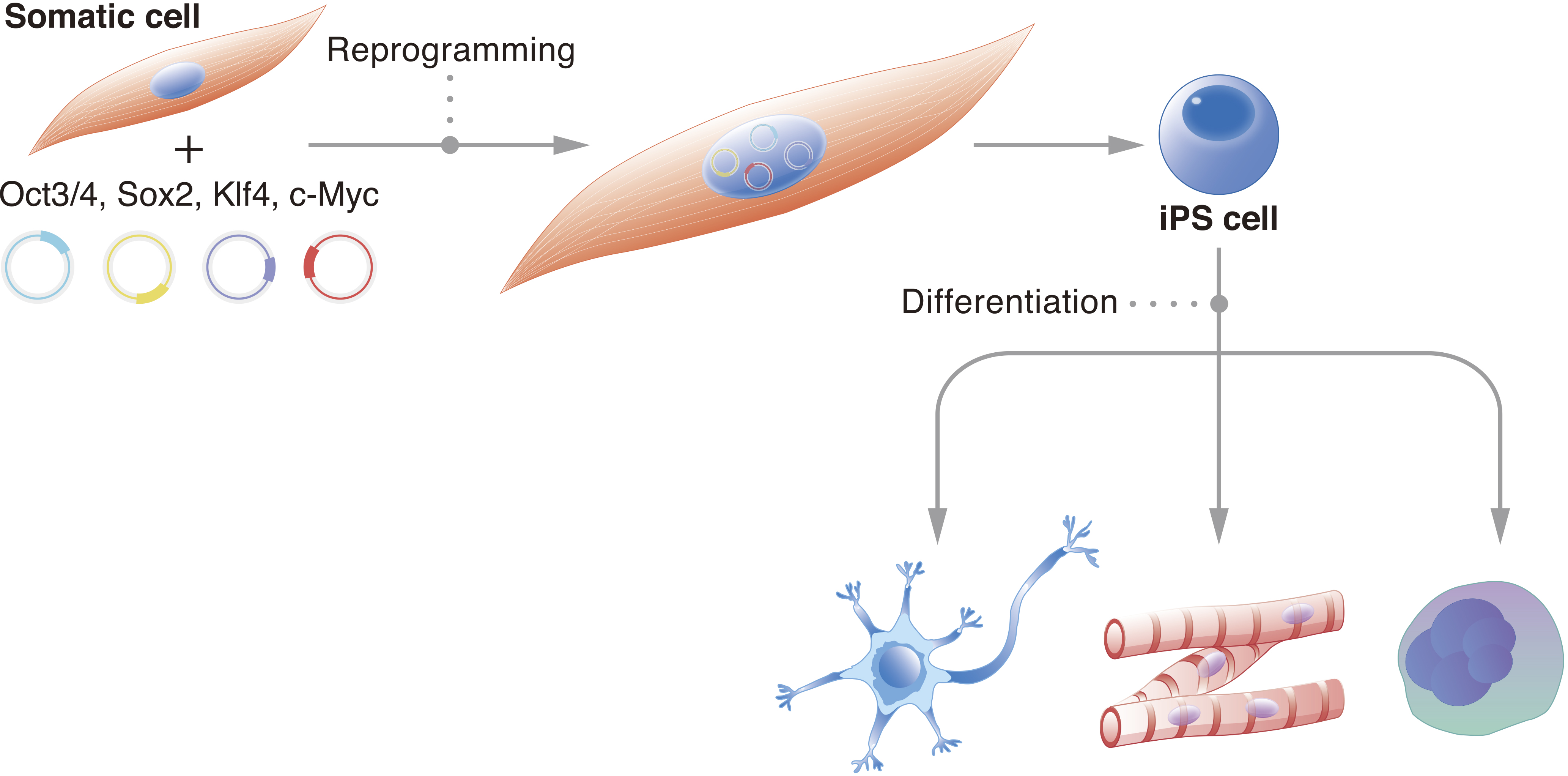
Figure 2. In iPSC banks such as the iPS Cell Stock for Regenerative Medicine, blood is taken from a super donor, i.e., a donor who is homozygous at the major HLA-A, HLA-B, and HLA-DRB1 loci. The cells are reprogrammed into clinical-grade iPSCs. The iPSCs are distributed to organizations that are conducting cell therapy. The use of super donor samples increases the probability of donor-recipient matching. In the figure, cells from the donor match two of the three patients, all of whom have different major HLA-A, HLA-B, and HLA-DRB1 haplotypes.
From: Bringing Induced Pluripotent Stem Cell Technology to the Bedside
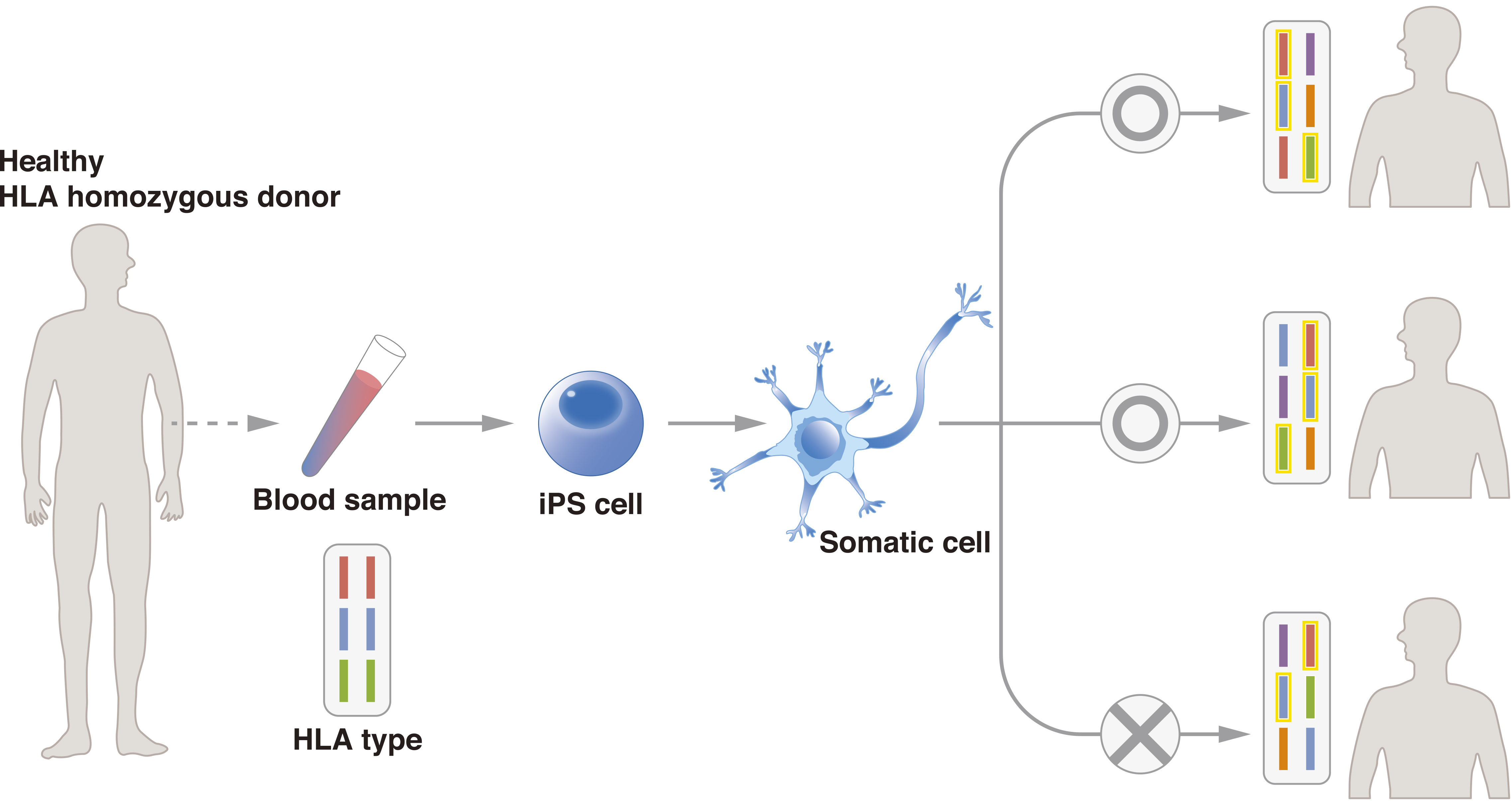
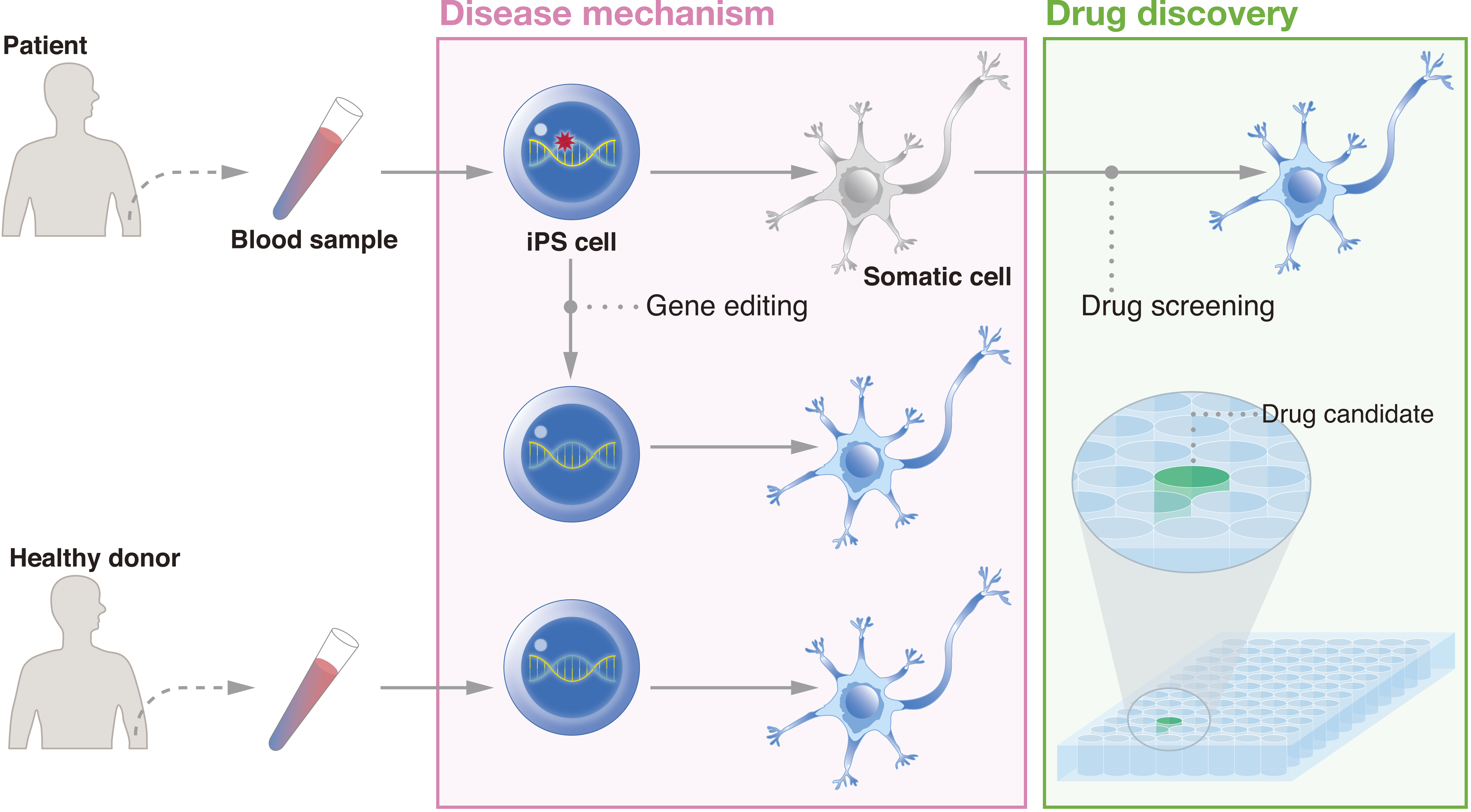
Figure 3. iPSCs provide a unique human model for drug discovery. Because patient cells can be reprogrammed to iPSCs and differentiated toward the diseased cell type, researchers can compare the differentiation process between patient cells and healthy donor cells and/or gene-corrected patient-derived cells to identify novel targets. Drug screening can be conducted to find candidates that alleviate the disease phenotype.
From: Bringing Induced Pluripotent Stem Cell Technology to the Bedside