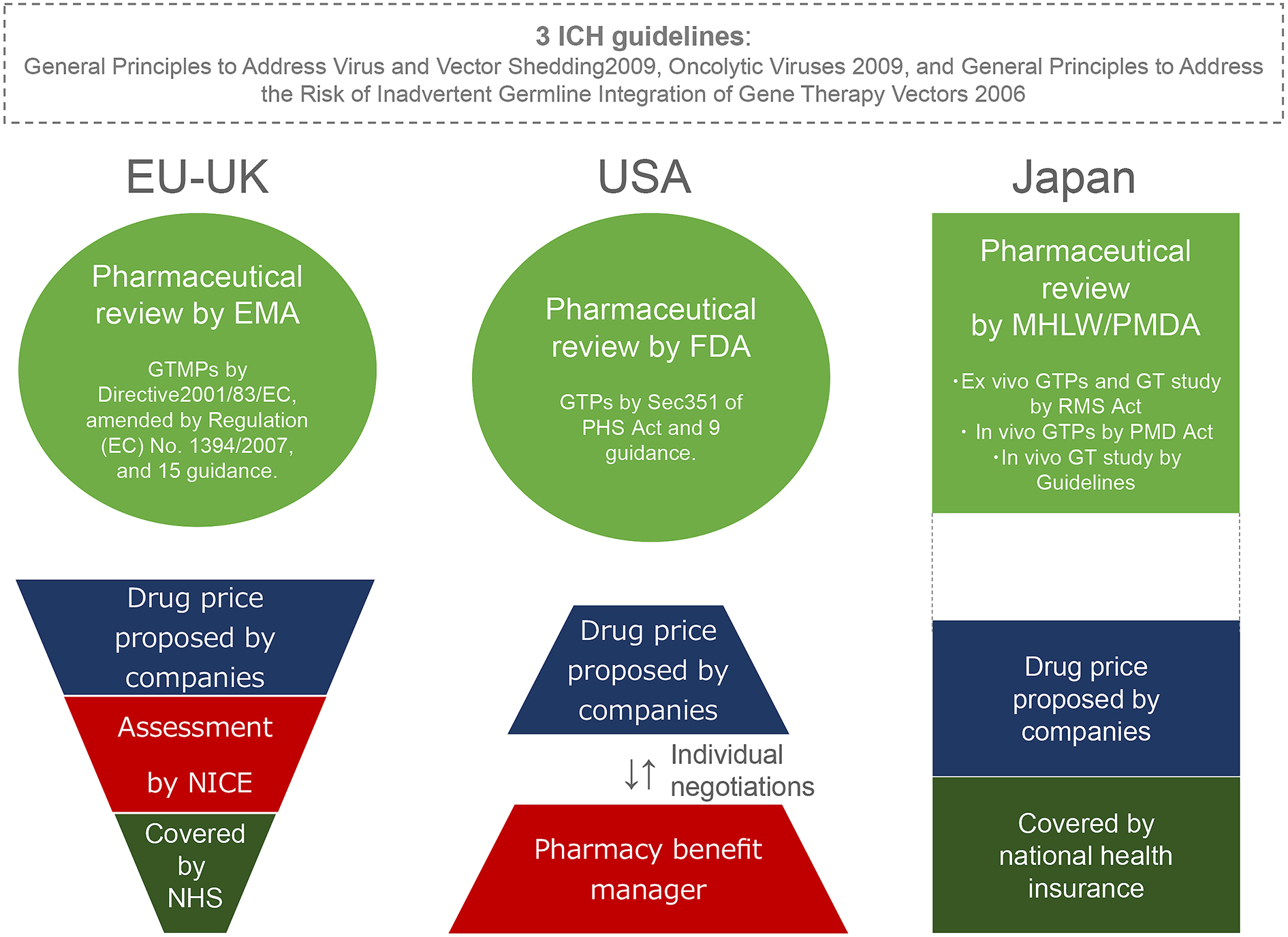
Figure 1. The regulatory approaches to, and pricing of, gene therapy products (GTPs) in the EU, the US and Japan.
The European Medicines Agency (EMA) regulates Gene Therapy Medicinal Products (GTMPs) as one of the Advanced Therapeutic Medicinal Products (ATMPs), including Somatic Cell Therapy Medicinal Products, Tissue Engineered Products, and Combined ATMPs (medical products combining one or more of the previous categories), under Directive 2001/83/EC, as amended by Regulation (EC) No. 1394/2007, with 15 guidance other than the three ICH guidelines. The US Food and Drug Administration (FDA) largely categorizes biologics into lower risk and higher risk products under the Public Health Services (PMS) Act. Lower risk products include human cells, tissues, and cellular and tissue-based products (HCT/Ps) that meet the criteria stipulated by Section 361 of the PMS Act: minimal manipulation; homologous use; not combined with another article (product); either no systemic effect and do not rely on metabolic effect of living cells; or have a systemic effect and rely on metabolic effect of living cells but are for autologous, first or second-degree blood relative or reproductive use. GTPs are classified as higher risk biologics that do not meet the criteria stipulated by Section 361. The EU and US regulate in vivo and ex vivo GTPs within the general regulatory framework, issuing instructive guidance, whereas Japan regulates gene therapy using three different regulatory tracks. If a GTP receives marketing approval from the EMA, the pharmaceutical company can negotiate the price with each EU member state. In the UK, the National Institute for Health and Care Excellence (NICE) assesses the price of a proposed drug based on the principle of cost-effectiveness. National Health Service coverage is only extended to drugs when NICE’s assessment of the price is favorable. In the US, pharmaceutical companies individually negotiate drug prices with pharmacy benefit managers based on economic liberalism. In Japan, the price of a drug that is to be covered by national health insurance is generally proposed by pharmaceutical companies. However, the cost-effectiveness will likely be considered in pharmaceutical pricing.
From: Raising Gene Therapy for Unmet Medical Needs in Japan