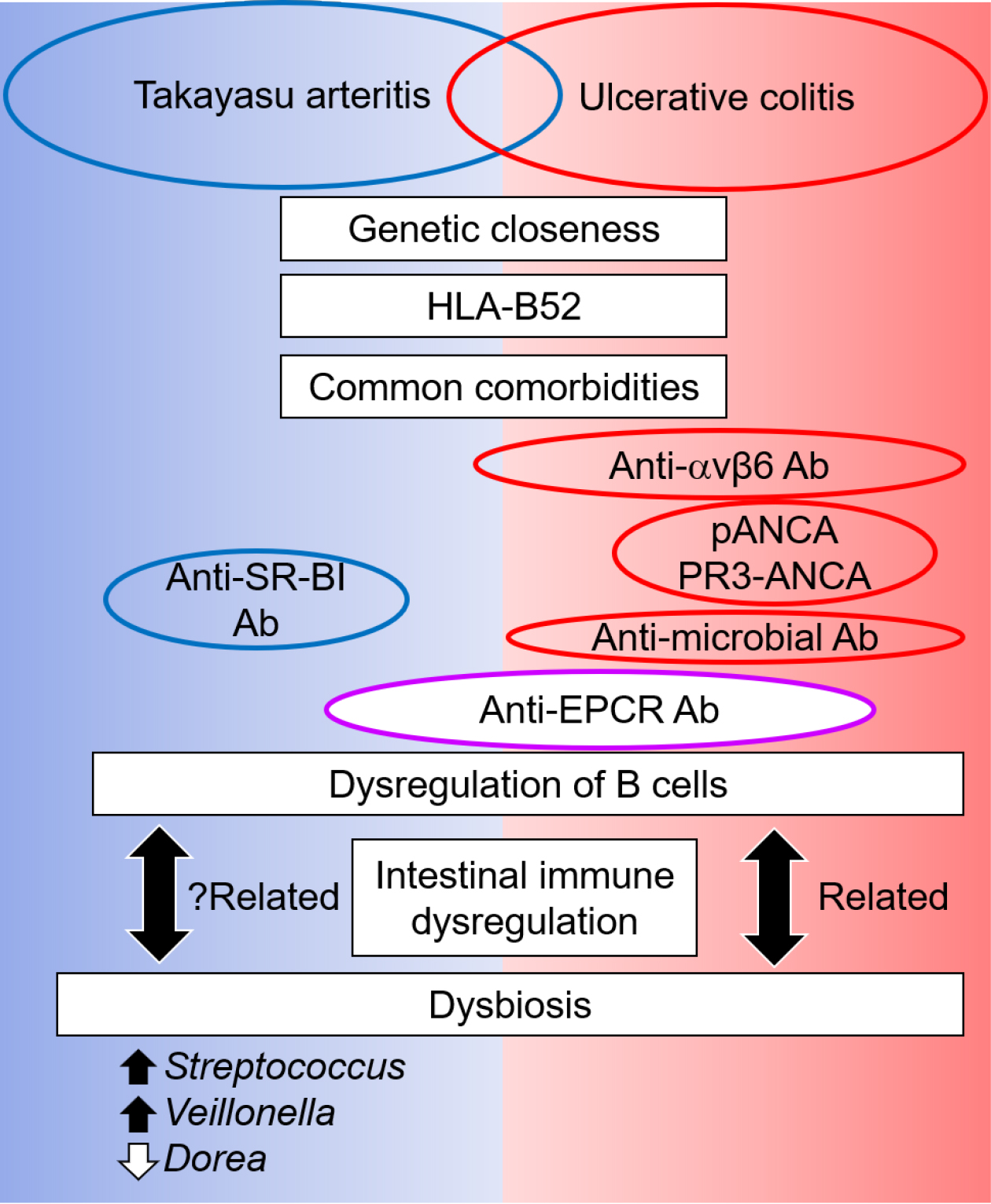
Figure 1. Suggested pathophysiology in Takayasu arteritis
Inflammation begins around the vasa vasorum, and the involvement of heat-shock protein 65 and MHC Class I chain-related A has been suggested. Although the first inflammatory process is unproven, cytotoxic activity by CD8+ T cells, γδ T cells, and natural killer cells, or the activation of dendritic cells (DC) and subsequent recruitment of immune cells by chemokines, seems to be the initial step. The recent discovery of anti-EPCR autoantibodies (Ab) and anti-SR-BI Ab suggests their roles in endothelial cell (EC) activation. Furthermore, anti-EC Ab (AECA) induces cytotoxicity, cytokine production, apoptosis, and mTOR pathway activation in EC. Subsequently, the recruited immune cells are activated. The activation of Th1 cells together with IL-12 and IL-18 secreted from DC releases tumor necrosis factor α and interferon γ, which activate macrophages, giant cells, and EC. The differentiation of Th17 cells is induced by IL-23, which is secreted by DC and macrophages. Anti-EPCR antibodies also promote Th17 cell differentiation, and Th17 cells promote neutrophil recruitment. Monocytes differentiate into macrophages and perform multiple effector functions. One of their functions is to promote inflammation by secreting chemokines, cytokines, reactive oxygen species (ROS), and reactive nitrogen intermediates (RNI). They also produce matrix metalloproteinase (MMP), which damages vascular components, and vascular endothelial growth factor (VEGF), which promotes neovascularization and subsequent inflammatory cell recruitment. Monocytes form granulomas together with neutrophils and T cells, and multinucleated giant cells are formed. Such inflammation disrupts the elastic lamellae, which contributes to artery dilatation. Giant cells and macrophages also produce growth factors, including platelet-derived growth factors and fibroblast growth factor-2 (FGF2), which trigger the migration of vascular smooth muscle cells into the intima, promote intimal proliferation of myofibroblasts, and produce matrix proteins, both of which contribute to artery stenosis. Tertiary lymphoid organs (TLOs) are formed in the adventitia, consisting of B cells, follicular dendritic cells (FDC), and high endothelial venules (HEV). Follicular helper T cells (Tfh) promote the maturation of B cells, and inflammation results in fibrous thickening and scarring of the adventitia.
Ab, autoantibody; AECA, anti-endothelial cell antibody; DC, dendritic cell; EC, endothelial cell; EPCR, endothelial protein C receptor; FDC, follicular dendritic cells; HEV, high endothelial venule; IL, interleukin; IFN-γ, interferon γ; MMP, matrix metalloproteinase; mTOR, mammalian target of rapamycin; RNI, reactive nitrogen intermediates; ROS, reactive oxygen species; SR-BI, scavenger receptor class B type 1; T, T cells; Tfh, follicular helper T cells; TLO, tertiary lymphoid organ; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
From: Common Autoantibody among Takayasu Arteritis and Ulcerative Colitis: A Possible Pathophysiology That Includes Gut-Vessel Connection in Vascular Inflammation
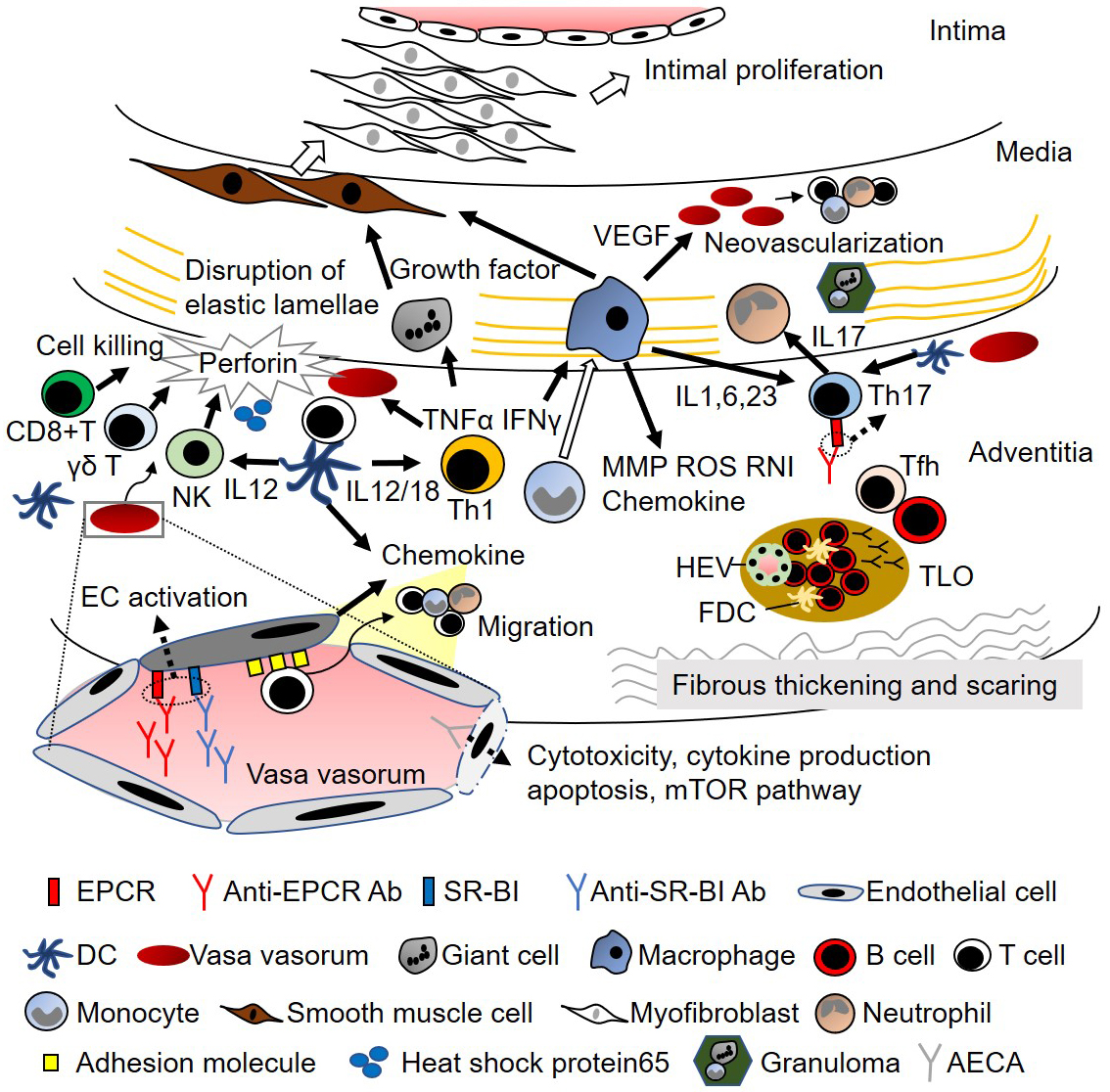
Figure 2. Comparison between Takayasu arteritis and ulcerative colitis: a possible role of gut-vessel connection in vascular inflammation
Takayasu arteritis and ulcerative colitis sometimes coexist and share genetic risks and comorbidities. Although different antibodies appear, anti-EPCR autoantibodies are present in both diseases, and B-cell dysregulation is increasingly known. Dysbiosis is a critical pathogenesis in ulcerative colitis and is related to the dysregulation of intestinal immunity and, subsequently, B cells. Dysbiosis is also present in Takayasu arteritis, and such common features of ulcerative colitis suggest a relationship between dysbiosis and immune dysregulation in Takayasu arteritis.
Ab, antibody; ANCA, anti-neutrophil cytoplasmic antibody; EPCR, endothelial protein C receptor; HLA, human leukocyte antigen; PR3, proteinase 3.
From: Common Autoantibody among Takayasu Arteritis and Ulcerative Colitis: A Possible Pathophysiology That Includes Gut-Vessel Connection in Vascular Inflammation