Corresponding author: Akihiro Umezawa, umezawa@1985.jukuin.keio.ac.jp
DOI: 10.31662/jmaj.2018-0029
Received: August 27, 2018
Accepted: May 29, 2020
Advance Publication: September 23, 2020
Published: October 15, 2020
Cite this article as:
Umezawa A, Sato Y, Kusakawa S, Amagase R, Akutsu H, Nakamura K, Kasahara M, Matsubara Y, Igarashi T. Research and Development Strategy for Future Embryonic Stem Cell-Based Therapy in Japan. JMA J. 2020;3(4):287-294.
Herewith, we review an updated progress of regenerative medical products using human embryonic stem cells (ESCs) in Japan. Two groups from Kyoto University and the National Center for Child Health and Development (NCCHD) established a novel derivation/cultivation system of ESCs for potential application in translational and clinical research. At the first stage of ESC derivation, murine feeder cells have been used in line with Japanese guidelines on public health associated with the implementation of the xenograft. To avoid exposure of ESCs to animal products in culture media, a xeno-free cultivating system has been established. Twelve ESCs (KhES-1, KhES-2, KhES-3, KhES-4, KhES-5, SEES-1, SEES-2, SEES-3, SEES-4, SEES-5, SEES-6, and SEES-7) are now available under a clinically relevant platform for industrially and clinically applicable regenerative medical products. NCCHD submitted an investigative new drug application to the Pharmaceuticals and Medical Devices Agency (PMDA) for using ESC-based products in patients with hyperammonemia due to genetic defects on March 2018 under the Pharmaceutical Affairs Law (now revised to the Pharmaceuticals, Medical Devices, and Other Therapeutic Products Act). Currently, up to ten ESC-based products are being prepared for intractable and rare disorders in Japan.
Key words: embryonic stem cells, ESCs, regulation, Pharmaceuticals, Medical Devices, and Other Therapeutic Products Act, The Act on the Safety of Regenerative Medicine
Human ESCs have high potential to be raw material to produce a variety of cell types due to their pluripotency when compared with somatic stem cells which have limited abilities in differentiation and self-renewal (1), (2). Once effective and efficient differentiation protocols are established to obtain a target cell, human ESCs are expected to stably supply a major portion of raw cell substrate materials used for manufacturing regenerative medical products. Kyoto University and the National Center for Child Health and Development (NCCHD) strictly complied with the Guidelines for Derivation and Distribution of Human Embryonic Stem Cells (3) and the Guidelines for Utilization of Human Embryonic Stem Cells (4) for human dignity to establish ESCs in Japan. In these two guidelines, the basic issues concerning the protection of personal information and protocols of derivation and usage of human ESCs are defined from the viewpoints of bioethics. The guidelines are now for development of novel treatments and pharmaceuticals/medical devices (5), (6).
Twelve human ESC lines (KhES-1, KhES-2, KhES-3, KhES-4, KhES-5, SEES-1, SEES-2, SEES-3, SEES-4, SEES-5, SEES-6, and SEES-7) are available as a resource of cell substrates to manufacture regenerative medical products at present for clinical usage under the Pharmaceuticals, Medical Devices, and Other Therapeutic Products Act (PMD Act, formerly Pharmaceutical Affairs Law) (7), (8), (9). KhES-1, KhES-2, and KhES-3 can be maintained in culture for up to 2 years without significant alteration in differentiated capability and karyotypes (7). Meanwhile, SEES-4, SEES-5, SEES-6, and SEES-7 can also be stably cultivated under xenogeneic-free conditions (9). SEES-1, SEES-2, and SEES-3 have been established by utilizing murine feeder cells. For a quality evaluation, we thus need to follow “Derivation and Characterization of Cell Substrates Used for the Production of Biotechnological/Biological Products,” “Guidelines on Public Health Infection Issues Accompanying Xenotransplantations,” and “Guidelines on Epithelial Regenerative Therapy Using 3T3J2 Strain or 3T3NIH Strain Cells as Feeder Cells” in order to prevent contamination of feeder cells and transmission of bacteria, fungi, viruses, and prions. KthES11, KthES12, KthES13, and KthES14 cells were established by Kyoto University in a feeder-free culture system to avoid contamination of murine cell components. Suemori has been generating KhESC lines at Kyoto University and has inserted a letter “t” to a name of newly established ESC, which are called KthES-11 cells, because he intends to apply KthES cells for therapeutic purpose in a clinical setting.
A new regulatory framework to supervise regenerative medicine, i.e., the “Act on the Safety of Regenerative Medicine” and the “Pharmaceuticals, Medical Devices, and Other Therapeutic Products Act,” was enacted in November 2015 (10). The Act on the Safety of Regenerative Medicine, in place of the Medical Practitioners Act and the Medical Care Act, provides regulations on medical professionals’ practices and clinical studies on regenerative medicine. Under the new “Act on the Safety of Regenerative Medicine,” regenerative medicine is classified into three categories depending on each therapy’s potential risk factor. The risk of regenerative medicine studies using ESCs is considered high; thus, ESC-based therapy will be placed in the high-risk medical technology category (Class I). Regardless of risk factor, all plans of using regenerative medicine must be submitted to the Ministry of Health, Labour and Welfare (MHLW). Moreover, before submitting plans to the MHLW, a certified special committee must submit their opinion on the preliminary plan created by the medical institution that intends to offer ESC-based therapy. This committee fulfills the legal accreditation criterion laid down by the MHLW. Once the committee gives its opinion, regardless of the recommendation, the medical institution may submit their preliminary plan for practicing regenerative medicine along with the committee’s opinion to the MHLW. In addition, medical institutions are obligated to conduct follow-up reports detailing the adverse effects and annual details about their regenerative medicine plan implementation, including the treatment provided, the number of patients treated, and the effectiveness of the treatment to the MHLW and the committee.
We follow two regulations for different legal benefits, namely, “safety and quality” (Guideline on Ensuring the Quality and Safety of Pharmaceuticals and Medical Devices Derived from the Processing of Human Embryonic Stem Cells) and “ethics” (Guidelines on the Derivation of Human Embryonic Stem Cells, Guidelines on the Distribution and Utilization of Human Embryonic Stem Cells) (1), (5), (6). These guidelines have different standpoints and, therefore, conflict: the former guideline focuses on protection of donors’ personal information and human dignity, and the latter prioritizes safety of raw materials.
If human ESC lines are established for the purpose of clinical use, donors need to be selected appropriately. Selection criteria and eligibility of the donors are important. Infection with hepatitis B virus, hepatitis C virus, human immunodeficiency virus, adult human T-lymphotropic virus, or parvovirus B19 shall be ruled out via physician-donor interviews and clinical laboratory tests, such as serological tests and nucleic acid amplification tests. Infection controls at the right time need to be done by retesting, taking into consideration the window period; however, such retesting is not practically allowed for ESCs according to the Guidelines on the Derivation of Human Embryonic Stem Cells (5). Infection with cytomegalovirus, Epstein-Barr virus, or West Nile virus shall also be ruled out, if necessary, via appropriate clinical laboratory tests. In addition, the eligibility of donors should be assessed whether he or she ever received a blood transfusion or underwent a transplantation procedure. Alternatively, it is conceivable to assess infectious pathogens at intermediate products during differentiation from the viewpoint of ethical and scientific rationality.
ESCs are considered the most undifferentiated cells similar to inner cells of blastocysts. Thus, the manufacturing process from undifferentiated ESCs to functional differentiated cells or final products is complicated and requires a long timeline, which may be challenging to perform. From the viewpoint of consistency and robustness, the most ideal foundation in the sustainable manufacture of ESC-based products is intermediate cell products/lines that have been well characterized (Figure 1). Preferably, the intermediate products should be stable per se and possess the ability to be propagated under relevant conditions. Furthermore, banks of the intermediate products should be renewed and must be able to differentiate properly into target cells. For certain final products, the proper establishment of sustainable intermediate cell products/lines as a cell bank at the intermediate stage of manufacturing process may be more significant and scientifically rational for the consistent manufacturing of desired safe products in addition to characterization, evaluation, or control of cells at the raw material stage. To minimize issues/concerns as much as possible, the most important concept and measures common to all types of biologics production are to ensure consistency and robustness in the manufacturing process. One of the core technical elements for proper and consistent production of biologics is to establish a base camp, i.e., to prepare production substrates at a relevant stage in the manufacturing process, which is applicable to extensive characterization and control, stable in quality, and from which constant processing to next intermediates and finally to a desired product is achievable.
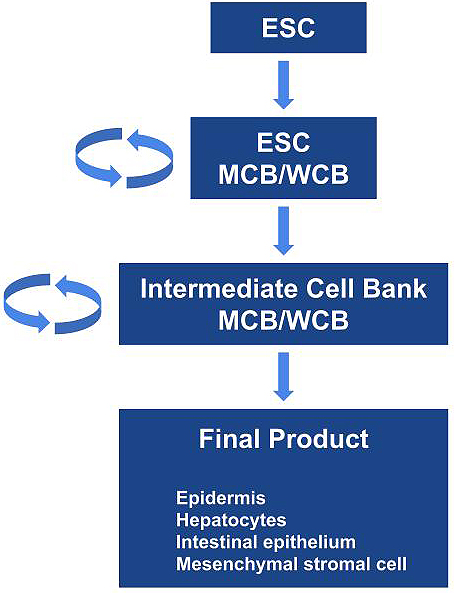
Human ESC-based products have been developed for clinical trials worldwide (11), (12), (13), (14), (15), (16), (17), (18), (19), (20). Clinical trials using hESC-derived cells for transplantation were identified by exploring the public database (Table 1). We used the search term “human embryonic stem cell” in reviewing “ClinicalTrials.gov,” from the US National Library of Medicine (https://clinicaltrials.gov/) database. We also evaluated original research articles and the sponsor’s websites on the respective clinical trials and/or transplant products for additional information. For example, ESC-derived products are being tested for use to patients with age-related macular degeneration, Stargardt disease, type 1 diabetes, and spinal cord injury in the United States; age-related macular degeneration and Stargardt disease in South Korea, the United Kingdom, China, and Brazil; age-related macular degeneration in Israel; severe heart failure in France; and Parkinson’s disease in Australia. We have filed a new investigational drug application to PMDA for ESC-based regenerative medical products to patients with congenital metabolic disorders and have ESC-derived mesenchymal stromal cells, epidermal cells, intestinal organoids, and hepatocytes in the development pipeline (21), (22).
Table 1. Clinical Trials Using hESC-derived Cells for Transplantation.
| Study title | Cell type (product name) | Sponsor/collaborator | Trial location | Disease | Stage of trial | Cell delivery | Status of trial | Registration number | Estimated or actual study completion date | Note/reference |
|---|---|---|---|---|---|---|---|---|---|---|
| A. Eye disease | ||||||||||
| Safety and Tolerability of Sub-retinal Transplantation of hESC-Derived RPE (MA09-hRPE) Cells in Patients with Advanced Dry Age-Related Macular Degeneration (Dry AMD) | hESC-derived RPE (MA09-hRPE) |
Astellas Institute for Regenerative Medicine | United States | Dry AMD | Phase I/II | Cell suspension | Completed | NCT01344993 | August 1, 2015 | < Progress reports > Lancet. 2015 Feb 7;385(9967):509-16. Lancet. 2012 Feb 25;379(9817):713-20. < Follow-up study > Invest Ophthalmol Vis Sci. 2016 Apr 1;57(5):ORSFc1-9. |
| Sub-retinal transplantation of hESC-derived RPE (MA09-hRPE) cells in patients with SMD | hESC-derived RPE (MA09-hRPE) | Astellas Institute for Regenerative Medicine | United States | Stargardt’s macular dystrophy (SMD) | Phase I/II | Cell suspension | Completed | NCT01345006 | August 1, 2015 | |
| Long Term Follow Up of Sub-retinal Transplantation of hESC Derived RPE Cells in Stargardt Macular Dystrophy Patients | hESC-derived RPE (MA09-hRPE) | Astellas Institute for Regenerative Medicine | United States | Stargardt’s macular dystrophy (SMD) | Phase I/II | Cell suspension | Active, not recruiting | NCT02445612 | December 1, 2019 | Follow-up study for NCT1345006 |
| Long Term Follow Up of Sub-retinal Transplantation of hESC Derived RPE Cells in Patients With AMD | hESC-derived RPE (MA09-hRPE) | Astellas Institute for Regenerative Medicine | United States | Dry AMD | Phase I/II | Cell suspension | Active, not recruiting | NCT02463344 | December 1, 2019 | Follow-up study for NCT1344993 |
| Safety and tolerability of sub-retinal transplantation of hESC-RPE cells in patients with SMD | hESC-derived RPE (MA09-hRPE) | Astellas Institute for Regenerative Medicine | United Kingdom | Stargardt’s macular dystrophy (SMD) | Phase I/II | Cell suspension | Completed | NCT01469832 | September 1, 2015 | < Progress reports > Ophthalmology. 2018 Nov;125(11):1765-1775. |
| A Follow up Study to Determine the Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With Stargardt's Macular Dystrophy (SMD) | hESC-derived RPE (MA09-hRPE) | Astellas Institute for Regenerative Medicine | United Kingdom | Stargardt’s macular dystrophy (SMD) | Phase I/II | Cell suspension | Active, not recruiting | NCT02941991 | December 1, 2019 | Follow-up study for NCT1469832 |
| A Phase 1b Dose Escalation Evaluation of Safety and Tolerability and a Phase 2 Proof of Concept Investigation of Efficacy and Safety of ASP7317 for Atrophy Secondary to Age-related Macular Degeneration | hESC-derived RPE (ASP7317) | Astellas Institute for Regenerative Medicine | United States | AMD | Phase I/II | Cell suspension | Recruiting | NCT03178149 | October 1, 2026 | Clinical study of ASP7317, a new cell line to replace MA09-Hrpe |
| A Phase I/IIa, Open-Label, Single-Center, Prospective Study to Determine the Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (MA09-hRPE) Cells in Patients With Advanced Dry Age-related Macular Degeneration (AMD) | hESC-derived RPE (MA09-hRPE) | CHABiotech (licensed from Astellas Institute) | Korea | Dry AMD | Phase I/II | Cell suspension | Unknown | NCT01674829 | April 1, 2016 | < Progress report > Stem Cell Reports. 2015 May 12;4(5):860-72. < Progress report of NCT01674829 > JAMA Ophthalmol. 2017 Mar 1;135(3):287-289. |
| Safety and Tolerability of MA09-hRPE Cells in Patients with Stargardt’s Macular Dystrophy (SMD) | hESC-derived RPE (MA09-hRPE) | CHABiotech (licensed from Astellas Institute) | Korea | Stargardt's macular dystrophy (SMD) | Phase I | Cell suspension | Unknown | NCT01625559 | June 1, 2015 | |
| A study of implantation of RPE in subjects with acute wet age-related macular degeneration | hESC-derived RPE (PF-05206388) | Pfizer/University College, London | United Kingdom | Wet AMD | Phase I | Membrane-immobilized monolayer sheet | Active, not recruiting | NCT01691261 | December 1, 2019 | < Progress report including nonclinical and clinical studies > Nat Biotechnol. 2018 Mar 19. doi: 10.1038/nbt.4114. |
| Retinal Pigment Epithelium Safety Study For Patients In B4711001 | hESC-derived RPE (PF-05206388) | Pfizer | United Kingdom | Wet AMD | Phase I | Membrane-immobilized monolayer sheet | Active, not recruiting | NCT03102138 | October 4, 2020 | Follow-up study for NCT01691261 |
| Safety and Efficacy Study of OpRegen for Treatment of Advanced Dry-Form Age-Related Macular Degeneration | hESC-derived RPE (OpRegen) | Lineage Cell Therapeutics (former BioTime)/Cell Cure Neurosciences | United States and Israel | Dry AMD | Phase I/II | Cell suspension | Recruiting | NCT02286089 | December 1, 2024 | https://lineagecell.com/products-pipeline/opregen/ < Nonclinical study > Transl Vis Sci Technol. 2017 Jun; 6(3): 17 |
| Study of subretinal implantation of human ESC-derived RPE cells in advanced dry AMD | hESC-derived RPE (CPCB-RPE1) | Regenerative Patch Technologies | United States | Dry AMD/geographic Atrophy | Phase I/II | Membrane- immobilized monolayer sheet | Active, not recruiting | NCT02590692 | June 1, 2023 | < Nonclinical study > Graefes Arch Clin Exp Ophthalmol. 2016 Aug;254(8):1553-65. < Progress report of clinical study (phase I/II) > Science Translational Medicine 04 Apr 2018: Vol. 10, Issue 435, eaao4097 http://www.sankeibiz.jp/business/news/180405/prl1804051101040-n1.htm |
| Clinical Study of Subretinal Transplantation of Human Embryo Stem Cell Derived Retinal Pigment Epitheliums in Treatment of Macular Degeneration Diseases | hESC-derived RPE | Southwest Hospital | China | AMD and Stargardt | Phase I/II | Cell suspension | Active, not recruiting | NCT02749734 | December 1, 2019 | No available information |
| Subretinal Transplantation of Retinal Pigment Epitheliums in Treatment of Age-related Macular Degeneration Diseases | hESC-derived RPE | Chinese Academy of Sciences/Beijing Tongren Hospital | China | Dry AMD | Phase I/II | Cell suspension | Recruiting | NCT02755428 | December 1, 2019 | No available information |
| Treatment of Dry Age Related Macular Degeneration Disease With Retinal Pigment Epithelium Derived From Human Embryonic Stem Cells | hESC-derived RPE | Chinese Academy of Sciences/The First Affiliated Hospital of Zhengzhou University | China | Dry AMD | Phase I/II | Cell suspension | Recruiting | NCT03046407 | December 1, 2020 | No available information |
| Stem Cell Therapy for Outer Retinal Degenerations | hESC-derived RPE | Federal University of São Paulo | Brazil | Dry AMD/wet AMD/Stargardt | Phase I/II | Cell suspension or monolayer in a polymeric substrate | Unknown | NCT02903576 | June 1, 2019 | No available information |
| A Safety surveillance study in subjects with macular degenerative disease treated with human ESC-derived retinal pigment epithelial cell therapy | hESC-derived RPE (MA09-hRPE) | Astellas Institute for Regenerative Medicine | United States | Macular degeneration | Phase I/II | Cell suspension | Enrolling by invitation | NCT03167203 | December 1, 2029 | A long-term (up to 15 years) safety surveillance study |
| Safety and Efficacy of Subretinal Transplantation of Clinical Human Embryonic Stem Cell Derived Retinal Pigment Epitheliums in Treatment of Retinitis Pigmentosa | hESC-derived RPE | Qi Zhou/Beijing Tongren Hospital | China | Retinitis pigmentosa | Phase I | - | Recruiting | NCT03944239 | December 1, 2020 | No available information |
| Interventional Study of Implantation of hESC-derived RPE in Patients With RP Due to Monogenic Mutation | hESC-derived RPE | Centre d'Etude des Cellules Souches | France | Retinitis pigmentosa (due to monogenic mutation) | Phase I/II | - | Recruiting | NCT03963154 | December 15, 2021 | No available information |
| B. Other disease | ||||||||||
| Transplantation of Human Embryonic Stem Cell-derived Progenitors in Severe Heart Failure (ESCORT) | hESC-derived CD15+ Isl-1+ cardiac progenitors | Assistance publique, Hôpitaux de Paris | France | Severe heart failure | Phase I | Cells embedded in fibrin patch | Completed | NCT02057900 | March 22, 2018 | < Nonclinical study > Eur Heart J. 2015 Mar 21;36(12):743-50. < Progress reports of clinical study > Eur Heart J. 2015 Aug 7;36(30):2011-7. J Am Coll Cardiol. 2018 Jan 30;71(4):429-438. |
| A Safety, Tolerability, and Efficacy Study of VC-01™ Combination Product in Subjects With Type I Diabetes Mellitus | hESC-derived pancreatic precursor cells (VC-01™ combination product) | ViaCyte/California Institute for Regenerative Medicine (CIRM) | United States/Canada | Type 1 diabetes | Phase I/II | PEC-01 cells encapsulated in a medical device | Active, not recruiting | NCT02239354 | January 1, 2021 | VC-01™, a combination product (PEC-01™ cells + Encaptra® DDS) (see PMID: 29369575) |
| One-Year Follow-up Safety Study in Subjects Previously Implanted With VC-01™ | hESC-derived pancreatic precursor cells (VC-01™ combination product) | ViaCyte | United States | Type 1 diabetes | Observational study | PEC-01 cells encapsulated in a medical device | Enrolling by invitation | NCT02939118 | November 1, 2021 | VC-01™ (PEC-Encap™) delivers the PEC-01 pancreatic progenitor cells in a immunoprotective device https://viacyte.com/products/pec%e2%80%90encap-vc-01 |
| A Safety and Tolerability Study of VC-02™ Combination Product in Subjects With Type 1 Diabetes Mellitus | hESC-derived pancreatic precursor cells (VC-02™ combination product, aka PEC-Direct) | ViaCyte | Canada | Type 1 diabetes | Phase I | PEC-01 cells loaded into a delivery device | Completed | NCT03162926 | February 15, 2018 | VC-02™ (PEC-Direct™) delivers the PEC-01 pancreatic progenitor cells in a non-immunoprotective device https://viacyte.com/products/pec-direct |
| A Safety, Tolerability, and Efficacy Study of VC-02™ Combination Product in Subjects With Type 1 Diabetes Mellitus and Hypoglycemia Unawareness | hESC-derived pancreatic precursor cells (VC-02™ combination product, aka PEC-Direct) | ViaCyte | United States | Type 1 diabetes | Phase I/II | PEC-01 cells loaded into a delivery device | Recruiting | NCT03163511 | March 1, 2022 | |
| Safety Study of GRNOPC1 in Spinal Cord Injury | hESC-derived oligodendrocyte progenitors (GRNOPC1/AST-OPC1) | Asterias Biotherapeutics | United States | Spinal cord injury | Phase I | Cell suspension | Completed (took over from Geron) | NCT01217008 | July 1, 2013 | < Nonclinical study > Regen Med. 2015 Nov;10(8):939-58. |
| Dose Escalation Study of AST-OPC1 in Spinal Cord Injury | hESC-derived oligodendrocyte progenitors (AST-OPC1) | Asterias Biotherapeutics | United States | Spinal cord injury | Phase I/II | Cell suspension | Completed | NCT02302157 | December 1, 2018 | https://www.cirm.ca.gov/our-progress/awards/phase-iiia-dose-escalation-safety-study-ast-opc1-patients-cervical-sensorimotor (You can check progress report on the trial) |
| A Study to Evaluate the Safety of Neural Stem Cells in Patients With Parkinson's Disease | Human parthenogenetic stem cell-derived neural stem cells(ISC-hpNSC) | Cyto Therapeutics/International Stem Cell Corporation | Australia | Parkinson's disease | Phase I | Cell suspension | Active, not recruiting | NCT02452723 | June 1, 2020 | < Nonclinical study > Sci Rep. 2016 Sep 30;6:34478. |
| Safety and Efficacy Study of Human ESC-Derived Neural Precursor Cells in the Treatment of Parkinson’s Disease | Human embryonic stem cells-derived neural precursor cells | Chinese Academy of Sciences/The First Affiliated Hospital of Zhengzhou University | China | Parkinson's Disease | Phase I/II | Cell suspension | Recruiting | NCT03119636 | December 1, 2020 | < Nonclinical study > Stem Cell Reports. 2018 Jul 10;11(1):171-182. |
| A Study to Evaluate Transplantation of Astrocytes Derived From Human Embryonic Stem Cells, in Patients With Amyotrophic Lateral Sclerosis (ALS) | Astrocytes derived from human embryonic stem cells (AstroRx) | Kadimastem | Israel | ALS (amyotrophic lateral sclerosis) | Phase I/II | Cell suspension | Recruiting | NCT03482050 | August 1, 2020 | < Nonclinical study > Stem Cell Res Ther. 2018; 9: 152. |
| Safety Observation on hESC Derived MSC Like Cell for the Meniscus Injury | hESC-derived MSC-like cell | Tongji Hospital/Chinese Academy of Sciences | China | Meniscus injury | Phase I | - | Active, not recruiting | NCT03839238 | September 30, 2020 | No available information |
| AMD: Age-related macular degeneration hESC: Human embryonic stem cell SMD: Stargardt's macular dystrophy RPE: Retinal pigment epithelium DDS: Drug delivery system |
||||||||||
None
Takashi Igarashi is the Deputy Editor of JMA Journal and on the journal's Editorial Staff. He was not involved in the editorial evaluation or decision to accept this article for publication at all.
Hayakawa T, Aoi T, Umezawa A, et al. A study on ensuring the quality and safety of pharmaceuticals and medical devices derived from processing of autologous human induced pluripotent stem(-like) cells. Regen Ther. 2015;2:81-94.
Akutsu H, Cowan CA, Melton D. Human embryonic stem cells. Methods Enzymol. 2006;418:78-92.
Guidelines on the derivation and distribution of human embryonic stem cells [Internet]. Ministry of Education, Culture, Sports, Science, and Technology (MEXT); 2009 [cited 2019 Aug 3]. Available from: https://www.lifescience.mext.go.jp/files/pdf/n743_00.pdf.
Guidelines on the utilization of human embryonic stem cells [Internet]. Ministry of Education, Culture, Sports, Science, and Technology (MEXT); 2009 [cited 2019 Aug 3]. Available from: http://www.lifescience.mext.go.jp/files/pdf/n743_01.pdf.
Guidelines on the derivation of human embryonic stem cells [Internet]. Ministry of Education, Culture, Sports, Science, and Technology (MEXT), the Ministry of Health, Labour and Welfare (MHLW); 2014 [cited 2019 Aug 3]. Available from: http://www.lifescience.mext.go.jp/files/pdf/n1553_01r2.pdf.
Guidelines on the distribution and utilization of human embryonic stem cells [Internet]. the Ministry of Education, Culture, Sports, Science and Technology (MEXT); 2014 [cited 2019 Aug 3]. Available from: http://www.lifescience.mext.go.jp/files/pdf/n1553_02r2.pdf.
Suemori H, Yasuchika K, Hasegawa K, et al. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem Biophys Res Commun. 2006;345(3):926-32.
Akutsu H, Nasu M, Morinaga S, et al. In vivo maturation of human embryonic stem cell-derived teratoma over time. Regen Ther. 2016;5:31-9.
Akutsu H, Machida M, Kanzaki S, et al. Xenogeneic-free defined conditions for derivation and expansion of human embryonic stem cells with mesenchymal stem cells. Regen Ther. 2015;1:18-29.
Azuma K, Yamanaka S. Recent policies that support clinical application of induced pluripotent stem cell-based regenerative therapies. Regen Ther. 2016;4:36-47.
NLM, NIH. ClinicalTrials.gov [Internet]. National Library of Medicine, National Institutes of Health [cited 2019 Aug 3]. Available from: https://clinicaltrials.gov/.
Kimbrel EA, Lanza R. Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov. 2015;14(10):681-92.
Kimbrel EA, Lanza R. Pluripotent stem cells: the last 10 years. Regen Med. 2016;11(8):831-47.
Ilic D, Devito L, Miere C, et al. Human embryonic and induced pluripotent stem cells in clinical trials. Br Med Bull. 2015;116:19-27.
Ilic D, Ogilvie C. Concise review: human embryonic stem cells-what have we done? What are we doing? Where are we going? Stem Cells. 2017;35(1):17-25.
Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11-22.
Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol. 2016;17(3):194-200.
Chichagova V, Hallam D, Collin J, et al. Cellular regeneration strategies for macular degeneration: past, present and future. Eye (Lond). 2018;32(5):946-71.
Martin U. Therapeutic application of pluripotent stem cells: challenges and risks. Front Med (Lausanne). 2017;4:229.
Guhr A, Kobold S, Seltmann S, et al. Recent trends in research with human pluripotent stem cells: impact of research and use of cell lines in experimental research and clinical trials. Stem Cell Rep. 2018;11(2):485-96.
Uchida H, Machida M, Miura T, et al. A xenogeneic-free system generating functional human gut organoids from pluripotent stem cells. JCI Insight. 2017;2(1):e86492.
Ando Y, Saito M, Machida M, et al. Can human embryonic stem cell-derived stromal cells serve a starting material for myoblasts? Stem Cells Int. 2017;2017:7541734.