Corresponding author: Taiga Nagase, taiganagase0418@gmail.com
DOI: 10.31662/jmaj.2021-0016
Received: February 9, 2021
Accepted: April 13, 2021
Advance Publication: July 6, 2021
Published: July 15, 2021
Cite this article as:
Nagase T, Wada S, Yokozawa T, Fujita A, Oda T. Bacteremia Caused by Both Legionella pneumophila Serogroup 2 and Helicobacter cinaedi. JMA J. 2021;4(3):297-301.
A 74-year-old woman with a history of pure red cell aplasia and hypogammaglobulinemia developed pneumonia. A urine antigen test and sputum subculture on buffered charcoal yeast extract (BCYE)α agar were positive for Legionella pneumophila. Serological testing identified L. pneumophila serogroup 2. An aerobic blood culture also became positive on day 5; its subculture on BCYEα agar revealed the same pathogen, but that on blood agar revealed Helicobacter cinaedi. We thus diagnosed her with bacteremia caused by both pathogens. Hence, in cases of H. cinaedi bacteremia along with pneumonia, the screening of other pathogens including L. pneumophila is needed.
Key words: Legionella pneumophila, Helicobacter cinaedi, pneumonia, bacteremia
Legionella pneumophila is a pathogen responsible for severe community-acquired or nosocomial pneumonia, especially among immunocompromised hosts or the elderly. It was first reported at the American Legion Convention in Philadelphia in 1976 (1), (2). L. pneumophila serogroup 1 is responsible for >80% of L. pneumophila pneumonia (3). A previous report showed that two of 127 isolates of L. pneumophila were of serogroup 2 (4). Regarding toxicity, there are no reports that serogroup 2 is more invasive than serogroup 1.
Helicobacter cinaedi, a Gram-negative spiral-shaped bacillus found in the gastrointestinal tract of humans and other animals, was first reported in 1985 in a patient infected with the human immunodeficiency virus (5). Bacteremia caused by H. cinaedi is reported frequently in immunocompromised hosts (6). However, reports on bacteremia caused by H. cinaedi or other pathogens are rare. To our knowledge, there is no report of bacteremia caused simultaneously by L. pneumophila and H. cinaedi. Here, we report the first such case.
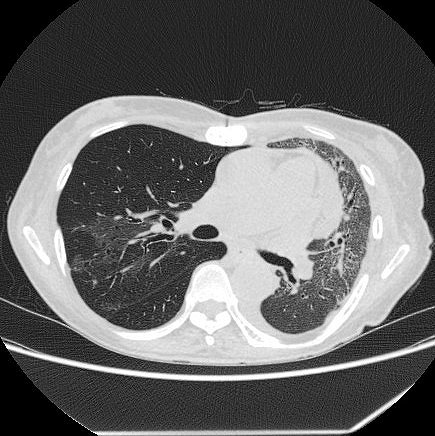
A 74-year-old woman was admitted to our emergency department with a history of pure red cell aplasia after thymectomy treated with cyclosporin and hypogammaglobulinemia treated with the regular administration of a gamma globulin preparation. Her chief complaint was wandering, which lasted for 3 days. At admission, she was alert, her respiratory rate was 24/min, blood pressure was 47/36 mmHg, heart rate was 186 bpm, and oxygen saturation was 92% with 8 L/min of oxygen. Rhonchi were heard in both lungs, and her tongue was dry and atrophic. The white blood cell count was 7.9 × 109/L, with neutrophil predominance (70%). Her hemoglobin level was 8.0 g/dL, serum urea nitrogen was 55.0 mg/dL, serum creatinine was 3.42 mg/dL, and C-reactive protein was 33.38 mg/dL (Table 1). Chest radiography revealed a decrease in permeability over her left lung (Figure 1), and chest computed tomography revealed infiltration with an air bronchogram over her left lung and a little pleural effusion bilaterally (Figure 2). She previously smoked a quarter pack per day for 5 years in her youth, but had no history of drinking. Twelve days before admission, she visited a hot spring. She had not used a humidifier or circulating bath. No other L. pneumophila infections were reported from that hot spring.
Table 1. Laboratory Data on Admission (Day 1).
| Hematology | Biochemistry | Coagulation | ||||||
|---|---|---|---|---|---|---|---|---|
| Hb | 8.0 | g/dL | TP | 4.9 | g/dL | PT-INR | 1.43 | |
| RBC | 252×104 | /μL | Alb | 2.5 | g/dL | APTT | 31.6 | s |
| Ht | 25.5 | % | T-Bil | 0.8 | mg/dL | |||
| PLT | 33.4×104 | /μL | ALP | 191 | U/L | |||
| WBC | 7,900 | /μL | AST | 30 | U/L | Arterial blood gas analysis | ||
| Neut | 70 | % | ALT | 17 | U/L | O2 8 L/minute reserver mask | ||
| Eos | 0 | % | LD | 470 | U/L | pH | 7.319 | |
| Bas | 1 | % | CK | 280 | U/L | pCO2 | 20.5 | mmHg |
| Mon | 9 | % | Na | 133 | mEq/L | HCO3- | 10.2 | mmol/L |
| Lym | 18 | % | K | 3.9 | mEq/L | pO2 | 85.4 | mmHg |
| RET | 11 | ‰ | Cl | 97 | mEq/L | Lac | 8.1 | mmol/L |
| BUN | 55.0 | mg/dL | ||||||
| CRE | 3.42 | mg/dL | ||||||
| CRP | 33.38 | mg/dL | ||||||
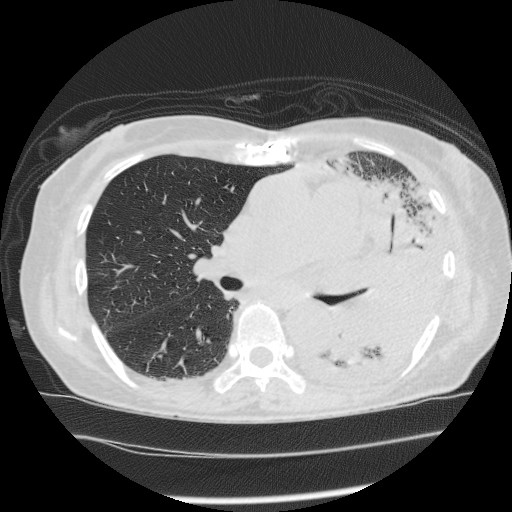
Based on clinical symptoms and imaging findings, pneumonia was suspected. We thus collected samples for sputum culture, blood culture, and urine antigen tests for Streptococcus pneumoniae and L. pneumophila. As the urine antigen test for L. pneumophila (RibotestⓇLegionella, Asahi Kasei Pharma Co., Tokyo, Japan) was positive, we diagnosed her with severe L. pneumophila pneumonia and began levofloxacin administration.
L. pneumophila was also found in sputum subculture on buffered charcoal yeast extract (BCYE)α agar under microaerobic conditions at 35°C, and slide agglutination tests with monovalent antisera identified L. pneumophila serogroup 2. An aerobic bottle of blood culture became positive on day 5, and subculture was performed on blood agar, chocolate agar, and BCYEα agar. Subculture on BCYEα agar revealed L. pneumophila under microaerobic conditions at 35°C, and slide agglutination tests with monovalent antisera identified L. pneumophila serogroup 2, whereas that on blood agar revealed H. cinaedi under the same conditions. We thus diagnosed her with bacteremia caused by both pathogens. We did not perform drug susceptibility tests for these pathogens and did not check for any mutations and toxicity changes.
The patient showed disturbances in consciousness, hypotension, and deoxygenation on day 2. She was intubated and transferred to the intensive care unit. Levofloxacin and tazobactam/piperacillin were administered with intensive care. She recovered gradually and was discharged from the hospital on day 60 (Figure 3 and 4).
In L. pneumophila pneumonia, the sensitivity of sputum culture is low (7), (8). Since 2019, a new serological test (RibotestⓇ Legionella) has been available in Japan with greater sensitivity for antigen detection in the urine. In some cases, L. pneumophila is not detected in sputum cultures and only urine tests for L. pneumophila are positive (9), (10). Although we cannot identify the serogroup in these cases, blood subculture on BCYEα agar might prove helpful and might make the diagnosis of L. pneumophila pneumonia more reliable. As L. pneumophila sometimes causes outbreaks, serogroup detection can contribute to epidemiological studies or infection control.
As L. pneumophila bacteremia is often reported among immunocompromised patients (9), blood subculture on BCYEα agar might be effective for detection. Some studies have reported the identification of L. pneumophila from a lung biopsy or bronchoalveolar lavage (9). Although the sensitivity of these tests might be high (7), these represent invasive techniques. In cases of pneumonia with a positive urine antigen test for L. pneumophila, blood subculture might allow for the identification L. pneumophila using a less invasive method.
Table 1 shows that the patient’s renal functions were decreased on admission. We assumed that prerenal renal dysfunction was caused by dehydration and renal dysfunction was caused by the extra-pulmonary symptoms of L. pneumophila pneumonia. We performed renal replacement therapy for several days, and her renal functions improved gradually.
It is possible that H. cinaedi was found as contamination. However, as she developed continuous bacteremia after discharge, it is more likely that bacterial translocation from her intestine occurred. In this case, H. cinaedi was the most probable cause of the positive signal in the automated blood culture machine. Without the urine antigen test for L. pneumophila, the possibility of L. pneumophila bacteremia would have been overlooked. To our knowledge, there is no report of H. cinaedi causing pneumonia. Thus, in cases of H. cinaedi bacteremia with pneumonia, the presence of other pathogens including L. pneumophila needs to be examined.
None
All authors were involved in data interpretation. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
All study participants provided informed consent.
Fraster DW, Tsai TR, Orenstein W, et al. Legionnaires’ disease: description of an epidemic of pneumonia. N Engl J Med. 1977;97(22):1189-97.
Marston BJ, Lipman HB, Breiman RF. Surveillance for Legionnaires’ disease. Risk factors for morbidity and mortality. Arch Intern Med. 1994;154(21):2417-22.
Yu VL, Plouffe JF, Pastoris MC, et al. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J Infect Dis. 2002;186(1):127-8.
Miyashita N, Higa F, Aoki Y, et al. Distribution of Legionella species and serogroups in patients with culture-confirmed Legionella pneumonia. J Infect Chemother. 2020;26(5):411-7.
Totten PA, Fennell CL, Tenover FC, et al. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J Infect Dis. 1985;151(1):131-9.
Kawamura Y, Tomida J, Morita Y, et al. Clinical and bacteriological characteristics of Helicobacter cinaedi infection. J Infect Chemother. 2014;20(9):517-26.
Yoshioka H, Takayanagi N, Ishiguro T, et al. Investigation of diagnostic method in 74 Legionella pneumonia cases. J Japan Soc Clin Microbiol. 2012;22(1):28-34.
Chen DJ, Procop GW, Vogel S, et al. Utility of PCR, culture, and antigen detection methods for diagnosis of Legionellosis. J Clin Microbiol. 2015;53(11):3474-7.
Jinno S, Pulido S, Pien BC. First reported United States case of Legionella pneumophila serogroup 1 pneumonia in a patient receiving anti-tumor necrosis factor-α therapy. Hawaii Med J. 2009;68(5):109-12.
Baldovin T, Pierobon A, Bertoncello C, et al. May car washing represent a risk factor for Legionella infection? Ann Ig. 2018;30(1):57-65.