Corresponding author: Riko Kato, katoriko7@gmail.com
DOI: 10.31662/jmaj.2021-0173
Received: September 15, 2021
Accepted: February 2, 2022
Advance Publication: March 25, 2022
Published: April 15, 2022
Cite this article as:
Kato R, Oguri M, Tsubata S, Adachi Y. Clinical Significance of Fecal Calprotectin for Evaluating Mucosal Inflammation with IgA Vasculitis. JMA J. 2022;5(2):277-279.
IgA vasculitis is the most common systemic small vasculitis in children. Its major clinical manifestations are palpable purpura, arthritis and arthralgias, gastrointestinal involvement, and renal manifestations. Regarding gastrointestinal manifestations, steroids are effective in reducing abdominal pain. However, exacerbation of gastrointestinal manifestation is frequently experienced when the steroid dose is being tapered. Thus, reliable biomarkers for gastrointestinal mucosal inflammation are needed. We report the case of a 4-year-old girl with abdominal-type IgA vasculitis. During the clinical course, we used several markers, such as fecal immunochemical test, fecal α1-antitrypsin and calprotectin. When fecal immunochemical test showed negative results and fecal α1-antitrypsin value returned to the normal range, corresponding to her abdominal pain improvement, fecal calprotectin levels remained high. This suggests that fecal calprotectin is more sensitive for evaluating mucosal inflammation than other markers. It could be a useful marker for mucosal inflammation in IgA vasculitis.
Key words: IgA vasculitis, alpha 1-antitrypsin, fecal calprotectin, fecal immunochemical test, inflammatory bowel disease
IgA vasculitis (IgAV) is the most common systemic small vasculitis in children. Palpable purpura and gastrointestinal (GI) involvement, arthritis, and renal involvement are major clinical manifestations. GI symptoms are seen in 50%-75% of patients (1). Although corticosteroids effectively reduce GI symptoms, there are no guidelines recommending the dose and duration of steroids. Patients are often treated empirically and experience recurrence of symptoms when the steroid dose is being tapered. Therefore, reliable biomarkers for GI mucosal inflammation are needed. Fecal calprotectin (FC) and fecal immunochemical test (FIT) have been widely used to monitor mucosal inflammation in inflammatory bowel diseases (IBD)(2), (3). We examined whether these markers could be used to evaluate GI mucosal inflammation while tapering steroids in a child with IgAV.
A 4-year-old girl was admitted with persistent abdominal pain and vomiting. A referring physician gave the patient probiotics; however, her symptoms did not improve. The patient had upper abdominal pain without guarding but did not have palpable purpura or arthritis. Laboratory results showed a white blood cell (WBC) count of 23.2 × 103/μL (reference range, 5.5-15.5 × 103/μL) and a C-reactive protein (CRP) level of 6.70 mg/dL (reference range, <0.3 mg/dL). Urine occult blood and protein levels were negative. An abdominal ultrasound showed marked wall thickening (4.1 mm) of the duodenum (Figure 1). Despite antibiotic treatment, the patient’s abdominal symptoms continued. On posthospitalization day 3, laboratory tests revealed a higher fibrin/fibrinogen degradation products level (24.2 μg/mL; reference range, <5.0 μg/mL) and decreased blood coagulation factor ⅩIII activity (34%; reference range, 70%-140%). We diagnosed the patient with abdominal-type IgAV i.e., only abdominal symptoms with no other clinical manifestations, and started to treat her with prednisolone (2 mg/kg/day).
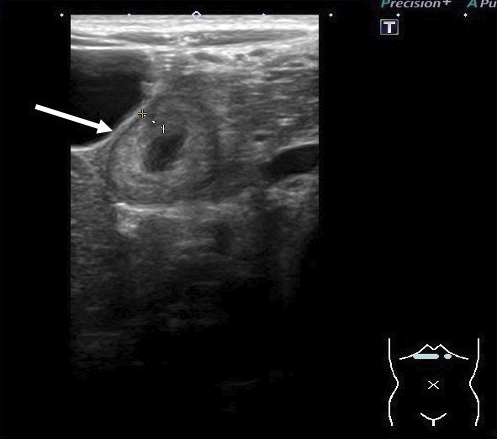
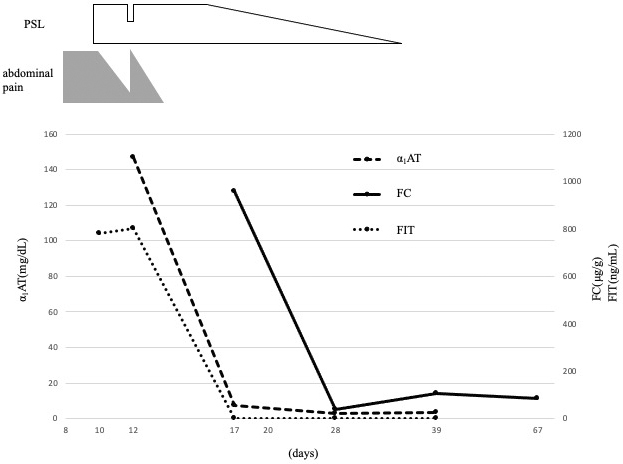
Her abdominal pain promptly improved after starting prednisolone, and an abdominal ultrasound showed improved duodenal wall thickness (DWT). After a 3-day course of prednisolone, we began tapering the dose from 2 mg/kg/day to 1 mg/kg/day. However, abdominal pain recurred on the first night of tapering; thus, we increased the dose back to 2 mg/kg/day. The steroid was gradually tapered over 30 days, and she had no recurrent abdominal pain.
To evaluate which test is most reliable for assessing GI mucosal inflammation in IgAV, we measured the FC, FIT, and fecal α1-antitrypsin (α1-AT) several times during the clinical course. During the first 7 days after hospitalization, even when the abdominal pain was initially improved by prednisolone, the levels of several markers, such as WBC, CRP, and α1-AT, remained high, and FIT was positive. After increasing the prednisolone dose again, her symptoms improved, and the WBC, CRP, and α1-AT levels returned to normal ranges. However, the FC level remained high (Figure 2). On day 21 of hospitalization, all markers, including FC, returned to normal ranges.
Although the palpable purpura is essential for diagnosing IgAV, it is not always the initial manifestation (1). Some cases were reported to have only GI symptoms without any purpura at their clinical course (4), (5). The European League against Rheumatism and Paediatric Rheumatology European Society recommends histological examination for the diagnosis of IgAV (6). We could not perform GI endoscopy because it was invasive for young children, so we had no histological diagnosis. However, it is reported that imaging studies and blood coagulation tests are helpful in identifying the thickening of the gastrointestinal wall, especially in the duodenum, and decreased coagulation factor ⅩIII activity in IgAV, respectively (7), (8). In our case, considering DWT and decreased coagulation factor XIII, we diagnosed the patient with abdominal-type IgAV.
FC, a mucosal inflammation marker for IBD, is a calcium-binding cytosolic protein located mainly in neutrophils and macrophages (2), (3), (9). It has been reported that FC increases in several acute abdominal diseases (9). There are few reports evaluating the clinical significance of FC in abdominal-type IgAV (9), (10). Teng et al. showed that an increase of FC in the early stage of acute phase IgAV tended to decrease after remission, reflecting the extent of intestinal inflammation (10). As a new finding in our case, FC concentration remained high even when the patient’s abdominal pain and DWT improved, and FIT and α1-AT became negative. After the FC level normalized, we could discontinue the steroid without recurrence of abdominal symptoms. This suggests that FC is more sensitive for evaluating mucosal inflammation than FIT and α1-AT. Further studies are warranted for its clinical application while tapering the corticosteroid.
None
We thank our patient and her parents for their consent to publish this case report. We also thank the radiologists for their support in the diagnosis and treatment.
R.K. designed this case report and drafted the initial manuscript. M.O. and S.T. provided technical support and conceptual advice. Y.A. reviewed and supervised the manuscript.
All authors have approved the final manuscript and agree to be accountable for all aspects of the manuscript.
This manuscript is a case report and does not need approval by IRB.
The patient and parents of the patient are aware of the intent to publish this case report and agree to it.
Rubino C, Paci M, Resti M, et al. Late relapse of Henoch-Schönlein purpura in an adolescent presenting as severe gastroduodenitis. Front Pediatr. 2018;6:355-9.
Siddiqui I, Majid H, Abid S. Update on clinical and research application of fecal biomarkers for gastrointestinal diseases. World J Gastrointest Pharmacol Ther. 2017;8(1):39-46.
Kato J, Hiraoka S, Nakarai A, et al. Fecal immunochemical test as a biomarker for inflammatory bowel diseases: can it rival fecal calprotectin? Intest Res. 2016;14(1):5-14.
Nathan K, Gunasekaran TS, Berman JH. Recurrent gastrointestinal Henoch-Schönlein purpura. J Clin Gastroenterol. 1999;29(1):86-9.
Gunasekaran TS, Berman J, Gonzales M. Duodenojejunitis: is it idiopathic or is it Henoch-Schönlein purpura without the purpura? J Pediatr Gastroenterol Nutr. 2000;30(1):22-8.
McCarthy HJ, Tizard EJ. Clinical practice: Diagnosis and management of Henoch-Schönlein purpura. Eur J Pediatr. 2010;169(6):643-50.
Ge Y, Liu S. Ultrasound, X-ray, computed tomography and clinical tests for diagnosis of abdominal purpura in children: a retrospective study. Exp Ther Med. 2020;19(6):3559-64.
Henriksson P, Hedner U, Nilsson IM. Factor XIII (fibrin stabilising factor) in Henoch-Schönlein’s purpura. Acta Paediatr Scand. 1977;66(3):273-7.
Kanik A, Baran M, Ince FD, et al. Faecal calprotectin levels in children with Henoch-Schönlein purpura: is this a new marker for gastrointestinal involvement? Eur J Gastroen Hepatol. 2015;27(3):254-8.
Teng X, Gao C, Sun M, et al. Clinical significance of fecal calprotectin for the early diagnosis of abdominal type of Henoch-Schonlein purpura in children. Clin Rheumatol. 2018;37(6):1667-73.