Corresponding author: Martin Susanto, martinsoesanto@yahoo.com
DOI: 10.31662/jmaj.2022-0202
Received: November 3, 2022
Accepted: January 16, 2023
Advance Publication: March 13, 2023
Published: April 14, 2023
Cite this article as:
Susanto M, Pangihutan Siahaan AM, Wirjomartani BA, Pardede W, Riantri I. Phenomenon of Hematocephalus: A Comprehensive Review of the Literature. JMA J. 2023;6(2):120-127.
The phenomenon of hematocephalus is still not fully understood. Intraventricular hemorrhage volume and intracranial pressure play a substantial role in the outcome and survival of the patients. The intraventricular hemorrhage resulting in an increased intracranial pressure is known by the term “hematocephalus.” The mortality rate ranges from 60% to 91% when hemorrhage affects all four ventricles. Even for partial hematocephalus, the mortality rate has been reported to be 32% to 44%. Therefore, the main objective in managing hematocephalus is to remove intraventricular blood efficiently and quickly because doing so will reduce ventricular dilatation and will rebalance cerebrospinal fluid circulation. However, the current standard management, which is inserting a ventricular drain immediately after an intraventricular hemorrhage, appeared to be of little value as the catheters are invariably clogged with blood clots. Long-term outcomes from the external ventricular drainage insertion plus subsequent intraventricular fibrinolytic therapy have been encouraging, but it also carries a substantial risk of new intracranial bleeding. The neuroendoscopic approach was created to aid in the treatment of hematocephalus and to enable the hematoma to be reduced or removed quickly without invasive surgery or the administration of fibrinolytic medications, preventing the intraventricular inflammatory reactions that result from hematoma degradation products. A controlled trial is necessary to ascertain whether this procedure enhances patient outcomes when compared to ventricular draining with or without thrombolysis.
Key words: hematocephalus, hematocephaly, hemocephalus, management
According to Benes, hematocephalus is an intraventricular hemorrhage (IVH) resulting in an increased intracranial pressure (ICP) (1). The phenomenon of hematocephalus is still not fully understood. Initially, it was considered only the elevation of ICP in the primary or purely intraventricular bleeding. However, later studies concluded that the true offending lesion was intracerebral bleeding to the deep brain structures, along with the shifting of these structures and brainstem compression (2). The accumulation of blood in the ventricles of the brain plus increased ICP is known as either hematocephalus (3), (4), hematocephaly (5), or hemocephalus (6). This terminology should not be confused with “hydrocephalus” as this condition caused by the buildup of cerebrospinal fluid (CSF) is not appropriate to be applied to patients who have IVH. However, hydrocephalus may develop in the long term after the ventricular blood has been evacuated because of either acute obstruction of the CSF pathways or chronic fibrosis of the leptomeninges (6).
To date, research regarding the phenomenon of hematocephalus is still limited. This comprehensive literature review aimed to provide information and collect research regarding the presentation, diagnosis, classification, mechanism, current neurosurgical treatment, and prognosis of hematocephalus.
We conducted extensive research in the database of PubMed, Google Scholar, EBSCO, DOAJ, and Cochrane. We utilized the terms “hematocephalus” OR “hematocephaly” OR “hemocephalus” to carry out a more thorough study. There were 274 articles found relevant. We took into account research in humans. As a result, we left out 210 studies. Specifically based on presentation, diagnosis, classification, mechanism, current neurosurgical treatment, and prognosis based on the research included in this review, we intended to make a thorough assessment of hematocephalus. Seven articles were excluded because of similarity to other articles, unclear statements, irrelevant correlation with the main topic, or concerning more on other diseases rather than hematocephalus. To add depth to the discussion material, we also added seven books of literature from medical dictionaries and textbooks. In the end, 64 studies were included in this comprehensive review.
The presence of IVH, which was once thought to be always deadly, has long been recognized by clinicians. This assumption was supported by the findings of postmortem studies, which revealed diffuse blood within the ventricular system in individuals with an apoplectic beginning of neurologic deterioration, advancing to coma, brainstem dysfunction, and death. Diagnosis may be performed on live patients with the advancement of computed tomography (CT) scanning, ultrasound sonography (USG), and magnetic resonance imaging (MRI) (7), (8), (9), (10).
The clinical discovery that primary intracerebral bleeding and cerebral aneurysm rupture frequently followed acute ventricular distension and substantially elevated ICP within a few hours of the ictus was made possible by breakthroughs in neuroradiology and evolving knowledge. The phenomenon substantially complicates the clinical course and is referred to as “acute hydrocephalus” whether or not blood is present in the ventricles. Numerous patients suffer from substantial ventricular bleeding, blood clot blockage, and ventricular tamponade. At autopsy, early acute ventricular distension would have been missed. Continuous CSF production can worsen transtentorial and transforaminal herniation as well as ventricular distension. In the past, many of these patients passed away too soon, with lumbar puncture and secondary pontine hemorrhage occasionally hastening their demise. The understanding of these processes and the neurosurgical management of acute ventricular dilation were vastly enhanced by more evidence. Although some people exhibit true acute hydrocephalus, the term acute hydrocephalus does not apply to those who frequently have IVH (6), (11), (12), (13), (14), (15), (16), (17).
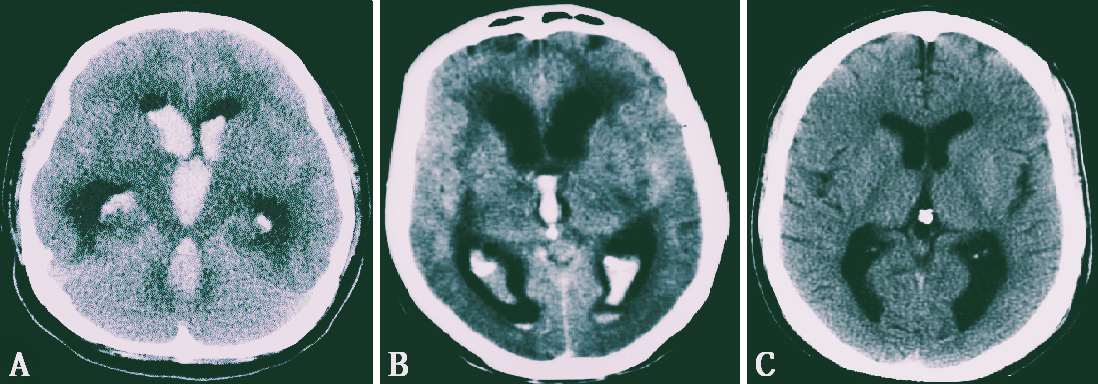
It is crucial to adhere to Pia’s proposed classification of IVH for diagnostic, therapeutic, and prognostic measures of hematocephalus (Figure 1) (18):
1) Hematocephalus totalis, which is hematoma throughout the entire ventricular system,
2) Hematocephalus partialis, which occurs when a blood clot only fills specific areas of the ventricular system,
3) IVH without real hematoma.
Using a 12-point grading scale, Graeb et al., who examined IVH, discovered a link between a high IVH grade and a negative outcome. The Graeb grading of IVH, which varies from 4 to 12, is the result of adding the component scores assigned to the lateral ventricles, third ventricle, and fourth ventricle (19):
a) Lateral ventricles (each lateral ventricle is scored separately).
Score 1 = trace of blood or mild bleeding
2 = less than half of the ventricle filled with blood
3 = more than half of the ventricle filled with blood
4 = ventricle filled with blood and expanded.
b) Third and fourth ventricles (each one is scored separately).
Score 1 = blood present, ventricle size normal
2 = ventricle filled with blood and expanded.
Hematocephalus results from bleeding into the cerebral ventricles, either from a main intraventricular source or, more commonly, from an intraparenchymal hemorrhage that is close to the ventricular wall, as in situations of hypertonic hemorrhage or aneurysm rupture (2), (20). Elevated ICP with decreased cerebral perfusion, mechanical disruption, ventricular wall distension, and perhaps an inflammatory response are the plausible processes by which IVH volume affects the outcome (21), (22), (23), (24), (25).
Hematocephalus used to be thought of as a clinical indication that was harmful or even foreboding. It quickly became clear, nevertheless that primary IVH did not pose a major risk to the individual because blood could be evacuated with a relatively easy puncture. Additionally, advancements in imaging technology made it possible to safely remove intracerebral blood clots in cases of subsequent intraventricular bleeding (2).
In cases of secondary hematocephalus, the impact of bleeding and clinical status during the acute stage of the disease depended on the amount of blood in the cerebral ventricles as well as the degree of cerebral injury brought on by the bleeding. Massive intraventricular bleeding causes the ventricular space to quickly fill with blood clots, making it impossible for the CSF hemolytic characteristics or the compliance of this fluid-filled region to function. Therefore, hydrocephalus and a subsequent progressive increase in ICP are caused by an abrupt stoppage of CSF circulation (2), (26).
Additionally, it has been proposed that the direct mass effect of IVH may result in decreased cerebral blood flow (10), (27). According to studies, the ependymal and subependymal layers of the brain and the brain stem sustain substantial damage as a result of the blood clot itself (28). According to animal studies, the damage to the surrounding tissues happens independently of the mass effect. The blood, most likely as a result of an inflammatory response, was discovered as the source of subarachnoid fibrosis, substantial ependymal cell loss, and subependymal glial proliferation on the walls of the lateral ventricles (29).
Previous studies have shown that IVH volume and ICP play a substantial role in outcome and survival (30), (31). Young et al. investigated the relationship between IVH volume and outcome, revealing that total ventricular volume (TVV) was associated with a poorer outcome and that there was a “lethal volume” of 20 mL (30). According to a different study, IVH volume predicts mortality irrespective of the Glasgow Coma Scale (GCS) (21). However, the challenge of measuring the volume of blood in standard clinical practice has hindered the study of IVH volume and its therapeutic application. IVH is more diffuse and encompasses various structures, in contrast to ICH, where the hematoma is more clearly defined and can be approximated using techniques like ABC/2 (22), (32).
Ziai et al. discovered that after controlling for IVH volumes, age, presentation GCS, and pulse pressure, the proportion of high ICP (>30 mm Hg) recordings per individual was an independent predictor of 30-day death (31). The relevance of ICP elevation (≥20 mm Hg) in the first 48 h following head injury as a poor prognostic predictor, particularly if ICP is recalcitrant to treatment, is consistent with the research on traumatic brain injury (31), (33), (34).
Because the compensatory mechanism is weakened, blood clots in the ventricular system cause abrupt CSF blockage and a marked rise in ICP. As the ascending reticular activating fiber system (ARAS) function is impaired by the presence of clots deep within the cortical or ventricular structures, an individual becomes ventilator dependent and needs a lengthy stay in the intensive care unit (ICU). This results in a decreased level of consciousness. The fibrin degradation products (FDPs) and bilirubin oxidation products (BOx) that are created during the breakdown process will enter and circulate in the CSF as the clot within the ventricular system undergoes lysis. Arachnoid granulation will then be reached by FDPs and BOx, which will result in delayed communicating hydrocephalus (35).
The juvenile thin-walled blood vessels that burst cause bleeding into the subependymal germinal matrix and possibly the lateral ventricles, which causes a condition known as germinal matrix hemorrhage (GMH). In a newborn rat GMH model, recent research showed the disruption of the exchange between CSF and interstitial fluid (ISF) and the development of hydrocephalus on day 28. These effects were partially restored by astrogliosis inhibition. GMH caused astrogliotic scarring, decreased CSF reabsorption through the meningeal lymphatic channels, and inhibited the exchange of CSF and ISF. Scar tissue development hinders the CSF clearance pathway. Glymphatic system dysfunction might be linked with aquaporin-4 redistribution. These waste products build up, as well as more ISF in the brain, as a result of glymphatic system disruption. The brain injury that results from the rupture of a significant blood artery is exacerbated by these occurrences, which are also present before and after subarachnoid bleeding (36), (37), (38).
The main objective in managing hematocephalus is to remove intraventricular blood efficiently and quickly because doing so will reduce ventricular dilatation and will rebalance the CSF circulation (39).
The current standard management, which is inserting a ventricular drain immediately after an IVH, appeared to be of little value as the catheters are invariably clogged with blood clots. However, only the sequelae of hydrocephalus and elevated ICP are addressed by this treatment (35), (40), (41), (42). Modern microsurgical, stereotactic, and drainage procedures cannot stop the resorption of blood degradation products from creating membranous structures or stenotic lesions in the cerebral ventricles. These mechanisms explain the presence of cysts and posthemorrhagic hydrocephalus (2).
Another less invasive treatment choice for individuals suffering from severe intraventricular bleeding is the placement of an external ventricular drainage system, followed by intraventricular fibrinolytic therapy with tissue plasminogen activator or urokinase. This approach showed a mortality rate of 6% and a poor-outcome rate of 30%. In cases of secondary IVH brought on by subarachnoid or intracerebral hemorrhage, this method has a success rate of more than 76%. However, when treating an IVH brought on by vascular malformations, this strategy could be risky. Hence, it is typically started only after stabilizing the abnormality with either an endoscopic procedure or an open surgery (10), (43), (44), (45), (46), (47), (48).
The fibrinolytic system is an enzyme mechanism that breaks down blood clots physiologically. In this system, plasminogen, an inactive proenzyme, can be transformed into plasmin, an active enzyme that breaks down fibrin into soluble FDPs. Tissue plasminogen activator, often known as t-PA, is the most substantial intrinsic plasminogen activator and is produced and secreted by endothelial cells (48). The unseen fibrinolytic property of CSF is responsible for the persistence of intracranial extravascular blood clots, particularly those in the ventricular system and subarachnoid region. Because t-PA is absent from normal CSF, there is no direct fibrinolytic activity. t-PA is released into the CSF as a result of meningeal irritation, such as bleeding, but the amounts are insufficient to effectively or quickly dissolve clots (49).
The quantity of blood in the ventricular system is visually estimated to determine the appropriate t-PA dose. The dosage ranges from 3 to 5 mg most frequently, with greater doses (8 mg) administered to comatose individuals whose IVH affects all four ventricles. When practical, a volume of CSF equal to the whole volume of t-PA being injected is withdrawn prior to the intraventricular injection of the drug. A 1 to 1.5 mL flush of preservative-free normal saline is administered after the injection. Then, the EVD is clamped for 2 h while the ICP is closely watched. The EVD is alternately opened and clamped for 1 h at a time for a total of 12 h after the initial 2 h (10).
Long-term outcomes from the intraventricular injection of fibrinolytic medicines have been encouraging, but it also carries a substantial risk of new intracranial bleeding. This strategy, meanwhile, is potentially risky when treating IVH brought on by vascular malformations, so it is typically employed only after stabilizing the abnormality with endoscopic or open surgery (39), (50), (51).
Different types of intracranial bleeding have been treated in the past using rigid or flexible endoscopes, but total blood clearing from all of the ventricles has never been achieved. Surprisingly, the most challenging and time-consuming element of the entire treatment is the aspiration of the clots in the lateral ventricles. The main issue is the necessity for orientation in an area that has been warped by the distension brought on by the hemorrhage without the use of visual cues. The most important orienting structure toward the third ventricle, the choroid plexus, must be visually located once a substantial amount of blood has been removed. The only tool that can navigate to the fourth ventricle is a flexible endoscope. When evaluating the advantages and disadvantages of rigid versus flexible devices, this is undoubtedly crucial. The aqueductal diameter is not an issue because it dilates naturally in all pathological circumstances and can be softly passed through without harming the nervous system. Because vision is relatively dim and hazy and movements inside the ventricles may result in significant cortical and subcortical damage, using rigid endoscopes to treat IVH is particularly damaging. The same logic applies to the microsurgical method, which is not recommended for the treatment of tetraventricular hemorrhage because of its intrusiveness and inability to reach all cavities, although it has potential for success in treating intraparenchymal hematomas (39), (52), (53), (54), (55), (56).
The technique of a neuroendoscopic approach has been suggested, nonetheless, in the idea that early evacuation of an intraventricular and intracerebral hematoma is beneficial (57), (58). The neuroendoscopic approach was created to aid in the treatment of hematocephalus and to enable the hematoma to be reduced or removed quickly without invasive surgery or the administration of fibrinolytic medications, preventing the intraventricular inflammatory reactions that result from hematoma degradation products (51). A time-limited procedure known as neuroendoscopic lavage allows the direct imaging of the ventricular system and any bleeding or blood clots while being carried out in sterile settings (59), (60). The ventricles remain dilated during the process because the intraventricular fluid volume is consistently maintained in balance. With this method, the hematoma itself can be decreased or removed through direct mobilization and aspiration, as well as liquefied blood, hematoma particles, and fibrin fragments. With the exception of the temporal horns, all ventricular compartments can be visualized and washed, and if necessary, draining catheters can be inserted using the neuroendoscope (Figure 2). Owing to a variety of factors, neuroendoscopic ventricular lavage may not always be sufficient. These include membrane and multiloculated hydrocephalus development, hemorrhage-induced impaired CSF resorption, concurrent or acquired aqueductal stenosis or blockage, partial hematoma clearance (residual in the temporal horns), and more. A controlled trial is necessary to ascertain whether this procedure enhances patient outcomes when compared to ventricular draining with or without thrombolysis (51).
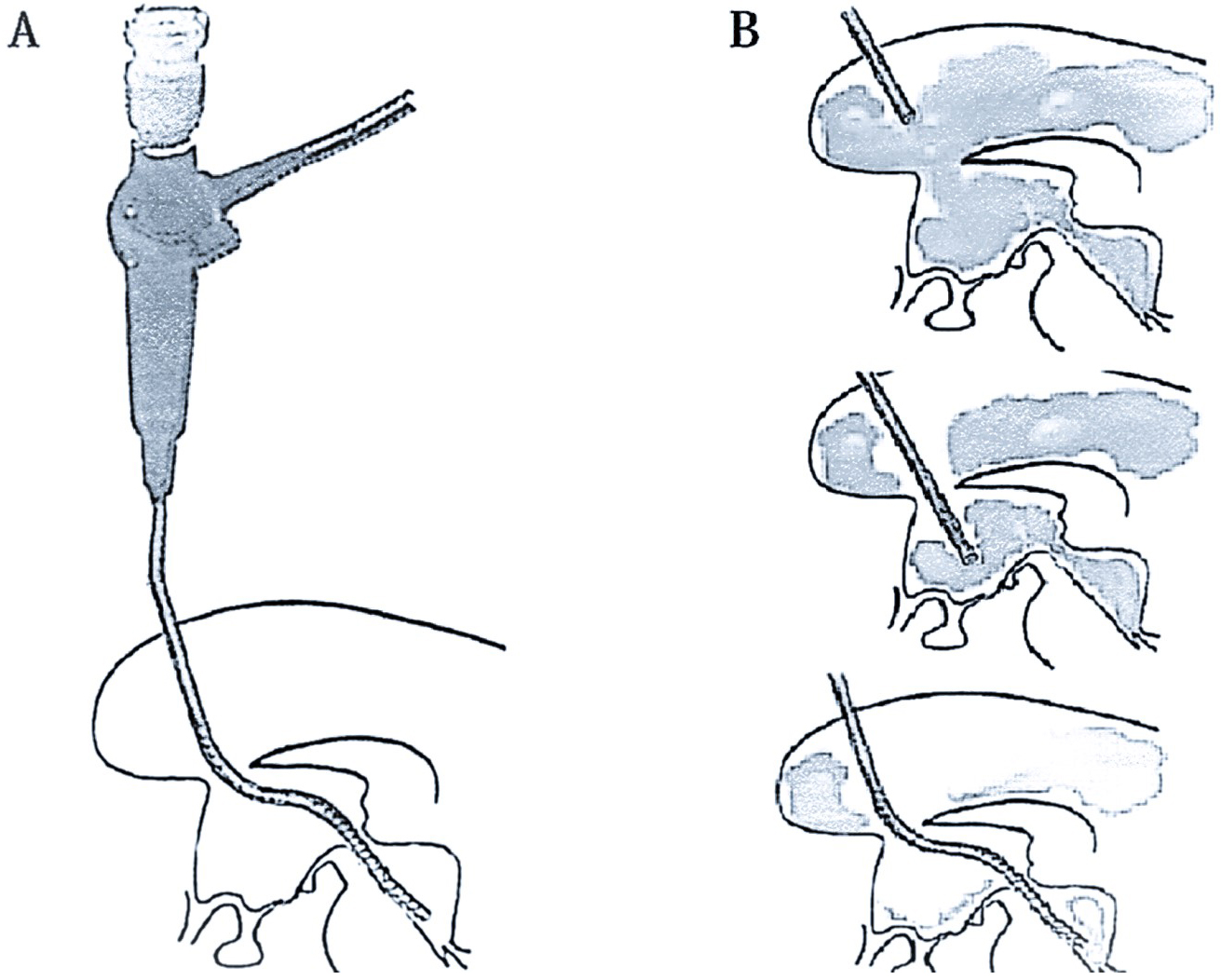
The application of neuroendoscopy in patients with massive intraventricular bleeding has substantially reduced drainage dependency and has produced positive clinical outcomes according to the research by Johnson et al. on the prevalence of shunt dependency and clinical outcomes in individuals with massive intraventricular bleeding treated with endoscopic washout and EVD. The early removal of hematomas, the development of new CSF diversion pathways, and the dilution of the FDPs and BOx that can impair the arachnoid granulations are possible causes of this. The modified Rankin scale score showed a substantial advantage in this investigation, suggesting that neuroendoscopy may produce a functional outcome that is potentially better (35).
Longatti et al. described treating 25 patients with hematocephalus totalis for removing blood clots in the cerebral ventricles using a flexible endoscope. On the day of admission, the endoscopic evacuation of the intraventricular blood was performed on the majority of the patients. Through the working channel of the endoscope, suction and irrigation were used to remove the clot from the lateral, third, and fourth ventricles. To remove the blood from the fourth ventricle, they maneuvered the endoscope through the Sylvius aqueduct. In all patients, they claimed to have been able to get rid of the majority of intraventricular blood. Postoperatively, ventricular drainage was performed for a median of 8 days. In 12% of patients, a ventriculoperitoneal shunt was required. There was no mortality associated with surgery. After the operation, the average stay in the ICU was 18 ± 12 days. The long-term mortality rate (>6 months) was 24%, whereas the short-term mortality rate (1 month) was 12%. 40% of patients resulted in full recovery (GCS score of 5) (39).
Novak et al. reported the outcomes of endoscopic treatment for primary and secondary hematocephalus in a group of 23 patients using the Wolf endoscopic system in conjunction with frame-based navigation in a virtual reality environment. The endoscopic third ventriculostomy was performed to reduce the amount of blood in the basal cisterns and to restore the patency of the CSF pathways, allowing lumbar puncture to be performed safely to facilitate the process of removing blood from the CSF in the majority of the treated patients. Septostomy was added to allow the revision of both lateral ventricles to be performed using a single approach. The clinical situation enhanced without the necessity for the placement of long-term drainage systems. One patient’s fatal outcome and the negative clinical outcomes in the other three individuals were unrelated to the endoscopic procedure (2).
According to a case study by Spazzapan et al., a neuroendoscopic ventricular lavage proved effective in treating a neonate with hematocephalus. The patient was born at 32 weeks gestation, weighed 1,740 g, had a head circumference of 31 cm, and achieved 9/10 on the Apgar score. An EVD was initially inserted 14 days after birth to relieve the hematocephalus and stop additional head enlargement due to the increasing head enlargement. After receiving treatment with the EVD for 14 days, the clot still existed and the neonate underwent a complete neuroendoscopic clot removal and lavage because the CSF was hemorrhagic. A ventriculosubgaleal shunt was left behind as a temporary drainage device after the endoscopic treatment went well. Within a week of surgery, the ventricular system began to gradually but steadily enlarge, necessitating repeated punctures of the subgaleal pocket before the CSF was sufficiently clear to allow the placement of a permanent ventriculoperitoneal drain (VPD). No signs of CSF infection were seen. The neonate was discharged after this treatment (51).
When hemorrhage extends into all four ventricles, the mortality rate has been 60% to 91%. Even for partial hematocephalus, the mortality rate has been reported to be 32% to 44%. According to Hallevi et al., there is a TVV cut-off of 40 mL above which individuals are 41 times more likely to have a poor outcome and a 50 mL “poor-outcome threshold” above which 100% of individuals have a poor outcome. Similar to other thresholds, TVV > 60 mL was established as the cut-off for mortality. However, the “lethal volume” at which TVV >60 mL had a 60% mortality rate was not identified. In general, the underlying disease, the location of the cerebral hematoma (in secondary IVH), and the severity of the intraventricular bleeding all have a substantial role in the morbidity and mortality of IVH individuals. Primary IVH survivors do frequently experience substantial morbidity. Compared to individuals with primary IVH, individuals with secondary IVH had worse outcomes. Individuals who have hematomas in the thalamus suffer the worst in terms of the location of the hematoma that is producing the IVH. The degree of ventricular dilatation, initial GCS, and elevated ICP are additional indicators of a poor outcome. With conservative therapy, the mortality rate for substantial intraventricular bleeding (Graeb >6) is close to 50%-75% (8), (9), (10), (19), (22), (30), (42), (44), (61), (62), (63), (64).
The phenomenon of hematocephalus is still not fully understood. The mortality rate ranges from 60% to 91% when hemorrhage affects all four ventricles. Even for partial hematocephalus, the mortality rate has been reported to be 32% to 44%. However, the current standard management, which is inserting a ventricular drain immediately after an IVH, appeared to be of little value as the catheters are invariably clogged with blood clots. Long-term outcomes from the EVD insertion plus subsequent intraventricular fibrinolytic therapy have been encouraging, but it also carries a substantial risk of new intracranial bleeding. The neuroendoscopic approach was created to aid in the treatment of hematocephalus and to enable the hematoma to be reduced or removed quickly without invasive surgery or the administration of fibrinolytic medications, preventing the intraventricular inflammatory reactions that result from hematoma degradation products. A controlled trial is necessary to ascertain whether this procedure enhances patient outcomes when compared to ventricular draining with or without thrombolysis.
None
The authors would like to thank PubMed, Google Scholar, EBSCO, DOAJ, and Cochrane for the accessibility and wide publication of the journals, which are collected and analyzed in this study.
All authors contributed to the study conception and design, performed data collection, made substantial contributions to the analyses and interpretation of the data, and wrote this manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
Not applicable.
Benes V. Ventricular haemorrhage. Zentralbl Neurochir. 1985;46(4):283-9.
Novak Z, Nadvornik P, Chrastina J, et al. Neuroendoscopic management of haematocephalus. Bratisl Lek Listy. 2005;106(3):114-8.
Dorland’s illustrated medical dictionary. 32nd edition. Philadelphia: W.B. Saunders Co.; 2012. 831 p.
Taber’s cyclopedic medical dictionary. 17th edition. Philadelphia: F.A. Davis Co.; 1993. 867 p.
Stedman’s medical dictionary. 27th edition. Philadelphia: Lippincott Williams & Wilkins; 2000. 796 p.
Stehbens WE. Pathology of the cerebral blood vessels. St. Louis: Mosby; 1972.
Caplan LR. Primary intraventricular hemorrhage. Case CS, Caplan LR. Intracerebral hemorrhage. Butterworth-Heinemann; 1994. p. 383-401.
Darby DG, Donnan GA, Saling MA, et al. Primary intraventricular hemorrhage: clinical and neuropsychological findings in a prospective stroke series. . Neurology. 1988;38(1):68-75.
Donauer E, Reif J, Al-Khalaf B, et al. Intraventricular hemorrhage caused by aneurysms and angiomas. Acta Neurochir. 1993;122(1-2):23-31.
Engelhard HH, Andrews CO, Slavin KV, et al. Current management of intraventricular hemorrhage. Surg Neurol. 2003;60(1):15-21 ;discussion 21.
Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke. 1998;29(7):1352-7.
Phan TG, Koh M, Vierkant RA, et al. Hydrocephalus is a determinant of early mortality in putaminal hemorrhage. Stroke. 2000;31(9):2157-62.
Kolluri VR, SenGupta RP. Symptomatic hydrocephalus following aneurysmal subarachnoid hemorrhage. Surg Neurol. 1984;21(4):402-4.
Milhorat TH. Acute hydrocephalus. N Engl J Med. 1970;283:857-9.
Milhorat TH. Intracerebral hemorrhage, acute hydrocephalus, and systemic hypertension. JAMA. 1971;218:221-5.
Milhorat TH. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1987;20:15-20.
Mohr G, Ferguson G, Khan M, et al. Intraventricular hemorrhage from ruptured aneurysm: Retrospective analysis of 91 cases. J Neurosurg. 1983;58:482-7.
Pia HW. The diagnosis and treatment of intraventricular haemorrhages. Prog Brain Res. 1968;30:463-70.
Graeb DA, Robertson WD, Lapointe JS, et al. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143:91-6.
Rosenow F, Hojer C, Meyer-Lohmann C, et al. Spontaneous intracerebral hemorrhage. Prognostic factors in 896 cases. Acta Neurol Scand. 1997;96:174-82.
Mayer SA, Thomas CE, Diamond BE. Asymmetry of intracranial hemodynamics as an indicator of mass effect in acute intracerebral hemorrhage. A transcranial Doppler study. Stroke. 1996;27:1788-92.
Hallevi H, Dar NS, Barreto AD, et al. The IVH score: a novel tool for estimating intraventricular hemorrhage volume: clinical and research implications. Crit Care Med. 2009;37:969-74.
Mayfrank L, Kissler J, Raoofi R, et al. Ventricular dilatation in experimental intraventricular hemorrhage in pigs: characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28:141-8.
Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res. 2007;1180:140-54.
Zhao X, Zhang Y, Strong R, et al. Distinct patterns of intracerebral hemorrhage-induced alterations in NF-kappa B subunit, iNOS, and COX-2 expression. J Neurochem. 2007;101:652-63.
Novak Z, Krupa P, Zlatos J, et al. Functions of the liquor space and its expansion. Bratisl Lek Listy. 2000;101:594-7.
Mayer SL, Kessler DB, Van Hurtum RL, et al. Effect of intraventricular blood on global cortical perfusion in acute intracerebral hemorrhage: a single photon emission computed tomographic study (abstract). Ann Neurol. 1995;38:228.
Andrews CO, Engelhard HH. Fibrinolytic therapy in intraventricular hemorrhage. Ann Pharmacother. 2001;35:1435-48.
Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: part 3. Neurosurgery. 1986;19:553-72.
Young WB, Lee KP, Pessin MS, et al. Prognostic significance of ventricular blood in supratentorial hemorrhage: a volumetric study. Neurology. 1990;40:616-9.
Ziai WC, Melnychuk E, Thompson CB, et al. Occurrence and impact of intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Crit Care Med. 2012;40:1601-8.
Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304-5.
Alberico AM, Ward JD, Choi SC, et al. Outcome after severe head injury. Relationship to mass lesions, diffuse injury, and ICP course in pediatric and adult patients. J Neurosurg. 1987;67:648-56.
Miller JD, Becker DP, Ward JD, et al. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977;47:503-16.
Johnson JR, Idris Z, Abdullah JM, et al. Prevalence of shunt dependency and clinical outcome in patients with massive intraventricular haemorrhage treated with endoscopic washout and external ventricular drainage. Malays J Med Sci. 2017;24:40-6.
Ye F, Keep RF, Hua Y, et al. Glymphatic system and post-hemorrhagic hydrocephalus. Brain Hemorrhages. 2023;4:44-6.
Ding Y, Zhang T, Wu G, et al. Astrogliosis inhibition attenuates hydrocephalus by increasing cerebrospinal fluid reabsorption through the glymphatic system after germinal matrix hemorrhage. Exp Neurol. 2019;320:113003.
Quintin S, Barpujari A, Mehkri Y, et al. The glymphatic system and subarachnoid hemorrhage: disruption and recovery. Explor Neuroprotective Ther. 2022;2(3):118-30.
Longatti P, Fiorindi A, Martinuzzi A. Neuroendoscopic aspiration of hematocephalus totalis: technical note. Neurosurgery. 2005;57:E409.
Little JR, Blomquist GA Jr, Ethier R. Intraventricular hemorrhage in adults. Surg Neurol. 1977;8:143-9.
Yamamoto Y, Waga S. Persistent intraventricular hematoma following ruptured aneurysm. Surg Neurol. 1982;17:301-3.
Shapiro SA, Campbell RL, Scully T. Hemorrhagic dilation on the fourth ventricle: an ominous predictor. J Neurosurg. 1994;80:805-9.
Findlay JM, Grace MG, Weir BK. Treatment of intraventricular hemorrhage with tissue plasminogen activator. Neurosurgery. 1993;32:941-7; discussion 947.
Mayfrank L, Rohde V, Gilsbach JM. Fibrinolytic therapy of intraventricular haemorrhage preceding surgical repair of ruptured aneurysms and arteriovenous malformations. Br J Neurosurg. 1999;13:128-31.
Nieuwkamp DJ, de Gans K, Rinkel GJ, et al. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000;247:117-21.
Rohde V, Schaller C, Hassler WE. Intraventricular recombinant tissue plasminogen activator for lysis of intraventricular hemorrhage. J Neurol Neurosurg Psychiatry. 1995;58:447-51.
Naff NJ, Carhuapoma JR, Williams MA, et al. Treatment of intraventricular hemorrhage with urokinase: effects on 30-day survival. Stroke. 2000;31:841-7.
Collen D, Lijnen HR. The fibrinolytic enzyme system. Sawaya R (ed). Fibrinolysis and the central nervous system. Hanley & Belfus; 1999. p. 14-25.
Findlay JM, Weir BK, Kanamaru K, et al. Intrathecal fibrinolytic therapy after subarachnoid hemorrhage: dosage study in a primate model and review of the literature. Can J Neurol Sci. 1989;16:28-40.
Todo T, Usui M, Takakura K. Treatment of severe intraventricular hemorrhage by intraventricular infusion of urokinase. J Neurosurg. 1991;4:81-6.
Spazzapan P, Bosnjak R, Velnar T. Ventricular lavage for pediatric haematocephalus treatment: a case report and operative technique. Surg Surg Endosc. 2020;2:37-44.
Auer LM. Endoscopic evacuation of intracerebral haemorrhage: high-tec-surgical treatment―a new approach to the problem? Acta Neurochir (Wien). 1985;74:124-8.
Horvath Z, Veto F, Balas I, et al. Biportal endoscopic removal of a primary intraventricular hematoma: case report. Minim Invasive Neurosurg. 2000;43:4-8.
Kamikawa S, Inui A, Kobayashi N, et al. Intraventricular hemorrhage in neonates: endoscopic findings and treatment by the use of our newly developed Yamadori-type 8 ventriculoscope. Minim Invasive Neurosurg. 2001;44:74-8.
Nakano T, Ohkuma H, Ebina K, et al. Neuroendoscopic surgery for intracerebral haemorrhage: comparison with traditional therapies. Minim Invasive Neurosurg. 2003;46:278-83.
Oka K, Go Y, Yamamoto M, et al. Experience with an ultrasonic aspirator in neuroendoscopy. Minim Invasive Neurosurg. 1999;42:32-4.
Schulz M, Bührer C, Pohl-Schickinger A, et al. Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. J Neurosurg Pediatr. 2014;13:626-35.
d’Arcangues C, Schulz M, Bührer C, et al. Extended experience with neuroendoscopic lavage for posthemorrhagic hydrocephalus in neonates. World Neurosurg. 2018;116:e217-24.
Brouwer AJ, Brouwer MJ, Groenendaal F, et al. European perspective on the diagnosis and treatment of posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal. 2012;97:F50-5.
Brouwer MJ, de Vries LS, Pistorius L, et al. Ultrasound measurements of the lateral ventricles in neonates: why, how and when? A systematic review. Acta Paediatr. 2010;99:1298-306.
Tuhrim S, Horowitz DR, Sacher M, et al. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617-21.
Findlay JM, Wong JH. Clinical aspects of intraventricular hemorrhage. Welch KMA, Caplan LR, Reis D, et al. Primer on cerebrovascular diseases. Academic Press; 1997. p. 437-46.
Jichici D, Frank JI. Thrombolytic therapy in neurointensive care. Crit Care Clin. 1997;13:201-27.
Mayfrank L, Lippitz B, Groth M, et al. Effect of recombinant tissue plasminogen activator on clot lysis and ventricular dilatation in the treatment of severe intraventricular haemorrhage. Acta Neurochir. 1993;122:32-8.