Corresponding author: Kei Nagai, knagai@md.tsukuba.ac.jp
DOI: 10.31662/jmaj.2023-0008
Received: January 19, 2023
Accepted: April 27, 2023
Advance Publication: June 30, 2023
Published: July 14, 2023
Cite this article as:
Nagai K, Harada T, Mase K, Iseki K, Moriyama T, Tsuruya K, Fujimoto S, Narita I, Konta T, Kondo M, Kasahara M, Shibagaki Y, Asahi K, Watanabe T, Yamagata K. Weight Loss Improves Liver Dysfunction and Dipstick Proteinuria in Obesity: The Japan Specific Health Checkups Study. JMA J. 2023;6(3):312-320.
Introduction: Obesity and inappropriate lifestyle is the major risk factors for liver dysfunction and proteinuria. Nevertheless, previous studies have not described the differential impacts of body weight changes and lifestyle modification on already developed liver dysfunction and proteinuria.
Methods: The original cohort was 933,490 individuals from the Japanese general population. In this investigation, we included 36,256 obese individuals with elevated levels of aspartate aminotransferase and/or alanine aminotransferase (≥31 IU/L) or positive proteinuria (+/− or more) in both the first and second years. Outcomes were the first normalization of these data defined as improvement in liver dysfunction and proteinuria. Times to outcomes were assessed using the Cox proportional hazards modeling for −1 kg/m2/year change in body mass index (BMI) changes in exercise and alcohol intake.
Results: The multivariable-adjusted hazard ratio (HR) for incident improvement in liver dysfunction with BMI change −1.0 kg/m2/year was 1.07 (95% confidence interval [CI] 1.05-1.09) in obesity and that with improved proteinuria was 1.04 (95%CI 1.02-1.07). Compared to subjects without exercise habits, subjects who gained exercise habits exhibited a higher rate of improvement in liver dysfunction (HR 1.08; 95%CI 1.01-1.15) but not in proteinuria (HR 0.98; 95%CI 0.88-1.08). Compared to subjects with continuous alcohol intake habits, subjects who quit alcohol intake also showed a higher rate of improvement in liver dysfunction (HR 1.20; 95%CI 1.09-1.32).
Conclusions: This study suggested that weight loss greater than 1 kg/m2/year improves liver dysfunction and dipstick proteinuria in obesity. Particularly, liver dysfunction can be remedied by acquiring an exercise habit and quitting alcohol intake.
Key words: liver disease, chronic kidney disease, body mass index, obesity, exercise
Obesity is considered to increase the risk of developing major risk factors for liver dysfunction due to several liver disorders, including fatty liver (1). Obesity is also involved in the development of chronic kidney disease (CKD) under pathologies, such as diabetes mellitus and hypertension (2). The presence of both liver dysfunction and proteinuria as a phenotype of CKD is associated with metabolic syndrome (3), (4). Recently, focus has been placed on extrahepatic diseases in patients with fatty liver, one of which is CKD (5). Metabolic dysfunction-associated fatty liver disease (MAFLD) is strongly and independently associated with a 1.34-fold risk of prevalent CKD and abnormal albuminuria (3). Awareness of the risk of obesity and education promoting a healthy lifestyle, including proper nutrition and exercise, may help prevent fatty liver disease and the onset of CKD (2), (6).
Weight loss is important to combat the disease burdens associated with metabolic syndrome, which has been increasing globally. Lifestyle modification to achieve weight loss is advocated as definitively beneficial for all patients with fatty liver disease (7). Liver dysfunction is considered to be alleviated after interventional body weight loss (8), (9), (10) and adoption of increased physical activity (10), (11), (12). Similarly, proteinuria is significantly decreased in obese CKD patients following proper interventional weight loss (13), (14). However, previous studies have not described the differential impacts of body weight changes and lifestyle modification on already developed liver dysfunction and proteinuria.
The Japan Specific Health Checkups study group has recently reported that weight loss may decrease the incidence of proteinuria (15). Few reports appear to have clarified the effects of changes in body weight and exercise habits on the development of liver dysfunction and proteinuria in an obese cohort of the Japanese general population. We hypothesized that improvements in lifestyle, particularly in terms of reducing body weight, acquiring exercise habits, and quitting alcohol intake, would improve liver dysfunction and proteinuria among obese patients. To test this hypothesis, we investigated a large observational cohort of subjects undergoing specific health checkups in Japan.
The original study cohort was based on 933,490 individuals from the general Japanese population who had participated in annual specific health checkups since 2008 according to “The Specific Health Check and Guidance in Japan.” Most study participants were therefore relatively healthy, community-dwelling residents aged between 40 and 74 years. Since this investigation required sequential information to determine changes in body mass index (BMI), liver function, and urinary dipstick protein, participants with results available from less than three examinations were omitted from analyses. Subjects for the first analysis thus comprised 591,671 individuals (58.8% women) for whom all data necessary for this study were available, that is, information regarding age and sex; consecutive results for BMI, systolic blood pressure, diastolic blood pressure, habitual smoking and alcohol intake, and uses of antihypertensive drugs, lipid-lowering drugs, and hypoglycemic drugs; and relevant laboratory data. Since the purpose of this study was to determine whether weight loss is associated with improvements in liver dysfunction and proteinuria, the analysis was further limited to the 36,256 obese individuals showing elevated levels of aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) (≥31IU/L each) or positive proteinuria in both the first and second years of the study, and we designated them as persistent liver dysfunction or persistent proteinuria (Figure 1). Of these final subjects, 27,615 had persistent liver dysfunction, 11,396 had persistent proteinuria, and 2,755 were duplicates. The database was used and managed solely by the statistician, and data from the study cohort were obtained only after concluding memoranda with the municipal heads. All data were anonymized. Information transfers were coordinated through local government officials, and the standard analytical file (SAF) version 4.0 was developed based on the approved study protocol. The original ethics approval was obtained from Fukushima Medical University (approval nos. #1485 and #2771) and the institutional review board for ethical issues at the University of Tsukuba (approval no. 999; UMIN: 000019774). Further analyses were then performed using the SAF without any personal identifiers.
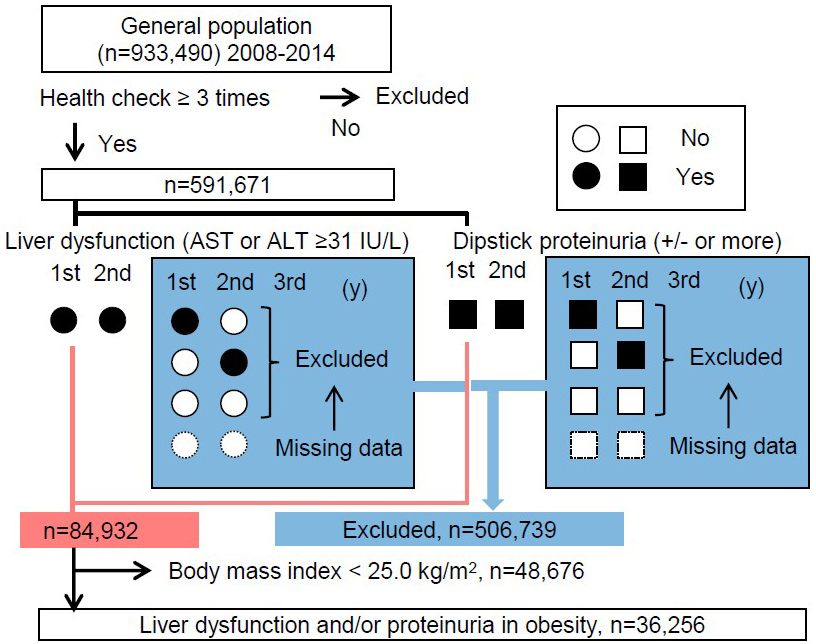
Urinalysis by the dipstick method was performed on a single-spot urine specimen. Urine dipstick results were interpreted by the medical staff at each local medical institution and recorded as (−), (+/−), (+), (2+), or (3+). In Japan, the Japanese Committee for Clinical Laboratory Standards (http://jccls.org/) has proposed that all urine dipstick results of (+/−) should correspond to a urinary protein level of 15 mg/dL. In this study, proteinuria was thus defined as a result of (+/−) or more to prioritize detection sensitivity. Blood samples were assayed within 24 h using an automatic clinical chemical analyzer after collection. Using the enzymatic method, serum creatinine was measured. Annual change in BMI (ΔBMI) was determined using data obtained from measurements in the first and second years and divided by the interval between measurements in years (ΔBMI per year).
The criteria for determining obesity differ from country to country, and the World Health Organization standard defines “obese” as a BMI of ≥30 kg/m2 (16). According to the standards set by the Japan Society for the Study of Obesity, we defined “obesity” in this study as a BMI of ≥25 kg/m2 (17). Based on our previous study, a reduction in BMI was defined as a change exceeding −1.0 kg/m2/year (15). The Specific Health Check includes a question regarding exercise habits using a binary question item: “Light sweaty exercise for at least 30 minutes at least 2 days a week for at least 1 year.” We utilized baseline and subsequent information to define “loss of habit” as a change from “yes” to “no” in each participant’s answers and “gain of habit” as a change from “no” to “yes.” Follow-up health checks were conducted through April 2015, as previously reported (18). Categorical variables are presented as numbers or percentages and continuous variables are presented as means and standard deviations (Table 1). Outcomes for analysis were the first normalization of liver dysfunction and/or proteinuria during follow-up (mean interval, 3.02 years from the second survey) among subpopulations divided by annual ΔBMI between the first and second surveys, with a mean interval of 1.2 years (Figure 1). This normalization of laboratory data changes is designated as “improvement” in this study. Times to outcomes were assessed using the Cox proportional hazards modeling for −1 kg/m2/year change in BMI and changes in exercise and alcohol intake with adjustment for age and sex, and further variables as follows: baseline BMI, estimated glomerular filtration rate, current smoking, current daily alcohol intake habit, systolic blood pressure, diastolic blood pressure, use of antihypertensive drugs, use of hypoglycemic drugs, use of lipid-lowering drugs, hemoglobin A1c, triglycerides, high-density lipoprotein, and low-density lipoprotein. Values of p < 0.05 were considered significant. Statistical analyses and graphical presentations were performed using SPSS version 27.
Table 1. Study Population with Liver Dysfunction and/or Proteinuria at Baseline.
| Liver dysfunction and/or proteinuria (+) | Obesity (−) | Obesity (+) | P value | |
|---|---|---|---|---|
| Study size | (persons) | 48,676 | 36,256 | |
| Sex | (% female) | 35.1 | 39.5 | <0.001 |
| Age | (years) | 63 ± 8 | 61 ± 8 | <0.001 |
| Body mass index | (kg/m2) | 22.1 ± 2.1 | 27.9 ± 2.6 | <0.001 |
| Systolic blood pressure | (mmHg) | 131 ± 18 | 136 ± 17 | <0.001 |
| Diastolic blood pressure | (mmHg) | 78 ± 11 | 81± 11 | <0.001 |
| Aspartate aminotransferase | (IU/L) | 35 ± 21 | 35 ± 18 | 0.004 |
| Alanine aminotransferase | (IU/L) | 34 ± 23 | 43 ± 25 | <0.001 |
| Baseline proteinuria, +/- or more | (% yes) | 31.0 | 39.9 | <0.001 |
| Estimated GFR | (mL/min/1.73 m2) | 75.4 ± 17.4 | 74.4 ± 17.2 | <0.001 |
| Free blood sugar | (mg/dL) | 101 ± 25 | 108 ± 28 | <0.001 |
| Hemoglobin A1c | (%) | 5.4 ± 0.8 | 5.7 ± 0.9 | <0.001 |
| Triglycerides | (mg/dL) | 139 ± 113 | 168 ± 116 | <0.001 |
| High-density lipoprotein | (mg/dL) | 62.3 ± 17.9 | 53.6 ± 13.3 | <0.001 |
| Low-density lipoprotein | (mg/dL) | 120 ± 34 | 129 ± 32 | <0.001 |
| Exercise habit | (% yes) | 36.4 | 42.0 | <0.001 |
| Smoking | (%) | 22.2 | 21.1 | <0.001 |
| Alcohol intake habit, daily | (% yes) | 35.4 | 36.4 | 0.003 |
| Use of antihypertensive drugs | (%) | 32.8 | 33.2 | 0.220 |
| Use of hypoglycemic drugs | (%) | 7.7 | 7.2 | 0.006 |
| Use of lipid-lowering drugs | (%) | 15.8 | 16.3 | 0.049 |
| ΔBMI | (kg/m2/year) | +0.1 ± 0.8 | -0.1 ± 1.0 | <0.001 |
| Time to endpoint | (days) | 1,088 ± 545 | 1,104 ± 549 | <0.001 |
| Abbreviations: BMI, body mass index; GFR, glomerular filtration rate. | ||||
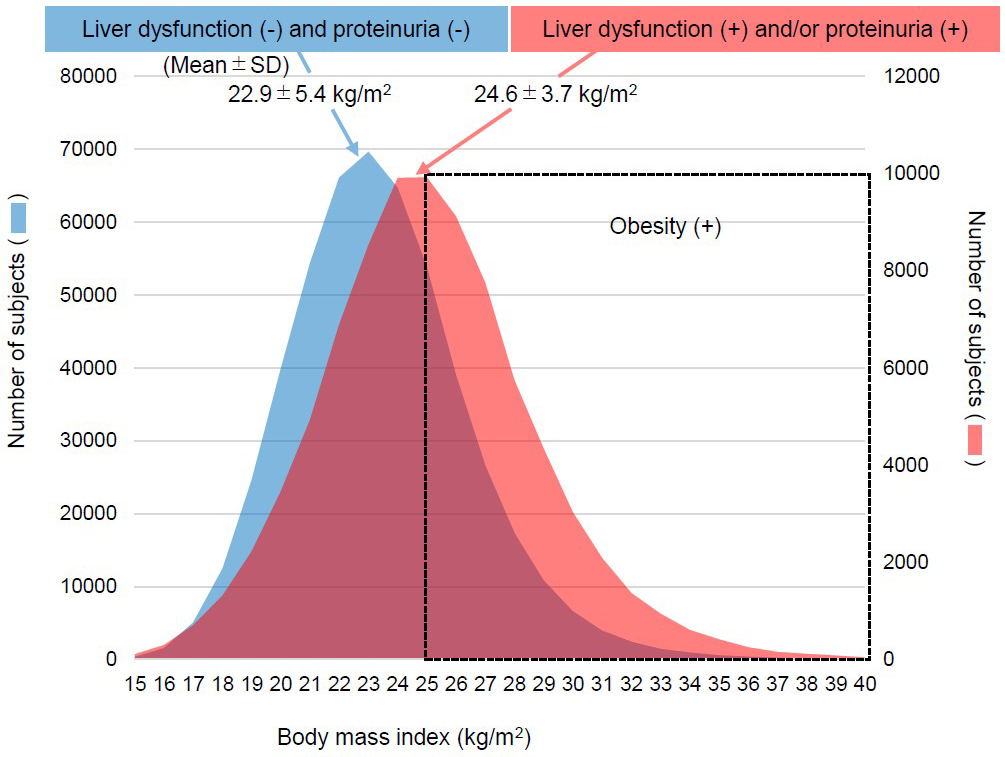
Among the total of 933,490 individuals who underwent specific health checkups, 591,671 individuals who participated in more than three checkups were identified (Figure 1). Next, we examined BMI at baseline for the 506,739 subjects to be excluded based on an absence of both liver dysfunction and proteinuria and for the 84,932 subjects with liver dysfunction or proteinuria. The mean BMI of subjects with liver dysfunction or proteinuria (24.6 ± 3.7 kg/m2) was much higher than that in patients with the absence of these conditions (22.9 ± 5.4 kg/m2, p < 0.001) (Figure 2). Finally, our analysis was further limited to the 36,256 obese cases with persistent liver dysfunction and/or persistent proteinuria. The included obese cases presented younger age than the excluded 48,676 non-obese patients, and worse profile for high ALT, hypertension, dyslipidemia, and glucose intolerance (Table 1). The improvement was achieved in 13,199 out of 27,615 subjects who had persistent liver dysfunction and was done in 5,329 out of 11,396 subjects who had persistent proteinuria during follow-up. Subjects who did not achieve the outcome were censored at the final observation.
Furthermore, to determine the possibility of an independent effect of reducing BMI in the obese population, we investigated these results using age- and sex-adjusted and multivariable factor-adjusted Cox proportional hazards modeling (Table 2). The age- and sex-adjusted hazard ratio (HR) for improvement in liver dysfunction with −1.0 kg/m2/year of BMI reduction was 1.07 (95% confidence interval [CI] 1.05-1.09) in the obese population and HR for improvement in proteinuria was 1.04 (95%CI 1.02-1.07). Multivariable-adjusted HRs were also significant, at 1.07 (95%CI 1.05-1.09) for improvement in liver dysfunction and 1.04 (95%CI 1.02-1.07) for improvement in proteinuria.
Table 2. Age- and Sex-Adjusted and Multivariable-Adjusted Rates of Improvement in Liver Dysfunction and Proteinuria in Obesity.
| Improvement of liver dysfunction | Improvement of proteinuria | ||||
|---|---|---|---|---|---|
| Model 1 | Units | Age- and sex-adjusted | P value | Age- and sex-adjusted | P value |
| Sex | female | 1.07 (1.03-1.11) | <0.01 | 1.33 (1.26-1.40) | <0.01 |
| Age | per −10 years | 0.89 (0.87-0.91) | <0.01 | 1.09 (1.05-1.13) | <0.01 |
| Δ Body mass index | per −1 kg/m2/year | 1.07 (1.05-1.09) | <0.01 | 1.04 (1.02-1.07) | <0.01 |
| Model 2 | Multivariable-adjusted | P value | Multivariable-adjusted | P value | |
| Sex | female | 1.05 (1.00-1.10) | 0.06 | 1.29 (1.20-1.40) | <0.01 |
| Age | per −10 years | 0.90 (0.87-0.93) | <0.01 | 1.00 (0.95-1.04) | 0.89 |
| Δ Body mass index | per −1 kg/m2/year | 1.07 (1.05-1.09) | <0.01 | 1.04 (1.02-1.07) | <0.01 |
| Body mass index | per −1 kg/m2 | 1.01 (1.00-1.02) | 0.03 | 1.01 (1.00-1.02) | 0.08 |
| Estimated GFR | per +10 mL/min/1.73 m2 | 0.97 (0.96-0.99) | <0.01 | 1.07 (1.06-1.09) | <0.01 |
| Smoking | no current smoking | 1.01 (0.96-1.07) | 0.59 | 1.08 (0.99-1.18) | 0.07 |
| Alcohol intake habit | not daily | 1.01 (0.99-1.04) | 0.32 | 1.01 (0.97-1.06) | 0.55 |
| Systolic blood pressure | per −10 mmHg | 0.98 (0.96-1.00) | 0.01 | 1.03 (1.01-1.05) | 0.01 |
| Diastolic blood pressure | per −10 mmHg | 1.02 (0.99-1.04) | 0.14 | 0.96 (0.93-1.00) | 0.05 |
| Use of antihypertensive drugs | no use | 1.07 (1.03-1.12) | <0.01 | 1.27 (1.19-1.35) | <0.01 |
| Use of hypoglycemic drugs | no use | 0.98 (0.91-1.05) | 0.54 | 1.17 (1.05-1.30) | <0.01 |
| Use of lipid-lowering drugs | no use | 0.99 (0.94-1.04) | 0.69 | 1.10 (1.02-1.19) | 0.02 |
| Hemoglobin A1c | per −1% | 0.96 (0.94-0.99) | 0.01 | 1.09 (1.05-1.13) | <0.01 |
| Triglycerides | per −10 mg/dL | 1.00 (1.00-1.00) | <0.01 | 1.00 (1.00-1.01) | 0.01 |
| High-density lipoprotein | per −10 mg/dL | 0.99 (0.97-1.01) | 0.20 | 1.00 (0.98-1.04) | 0.42 |
| Low-density lipoprotein | per −10 mg/dL | 0.99 (0.98-0.99) | <0.01 | 1.01 (1.00-1.02) | 0.22 |
| Cox proportional hazards model for −1 kg/m2/year change in BMI with adjustment for age and sex (Model 1), and further variables included as indicated in the table (Model 2). Abbreviations: GFR, glomerular filtration rate. | |||||
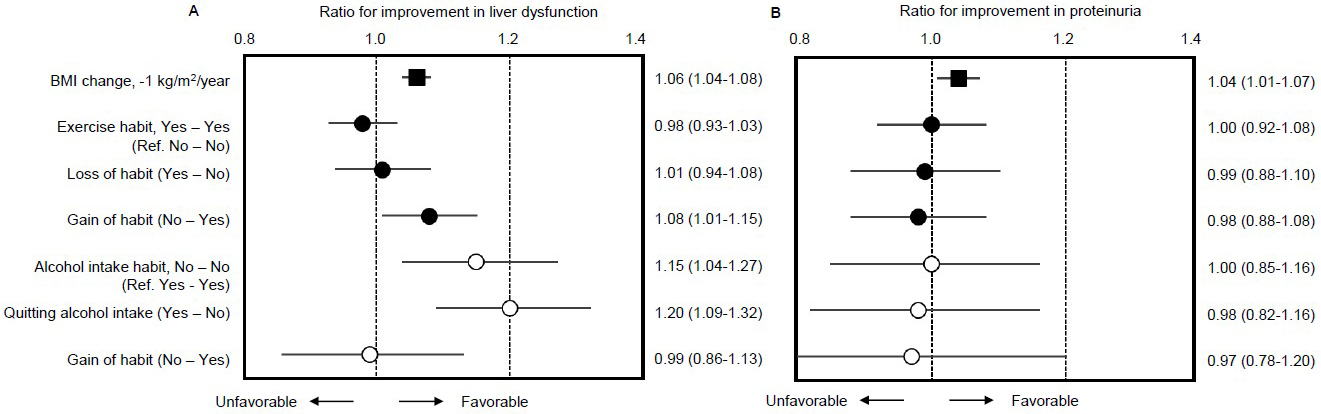
Finally, we examined the HRs of subjects with changes in exercise and alcohol intake as lifestyle factors using a multivariable-adjusted model (Figure 3A and 3B). Compared to subjects lacking exercise habits in both the first and second surveys (designated as “No-No”), subjects who gained an exercise habit (“No-Yes”) exhibited a higher rate of improvement in liver dysfunction (HR 1.08; 95%CI 1.01-1.15). Compared to subjects with continuous alcohol intake habits (“Yes-Yes”), subjects who quit alcohol intake (“Yes-No”) also showed a higher rate of improvement (HR 1.20; 95%CI 1.09-1.32) (Figure 3A). However, inconsistent with BMI change as a positive control for improvement in proteinuria (HR 1.04; 95%CI 1.01-1.07), neither gaining an exercise habit (HR 0.98; 95%CI 0.88-1.08) nor quitting alcohol intake (HR 0.98; 95%CI 0.82-1.16) impacted improvement in proteinuria within the mean 3.02 years of follow-up in this study (Figure 3B).
The worldwide prevalence of overweight and obesity is both high and increasing (19). Additionally, metabolic syndrome is commonly associated with both chronic liver disease and CKD presenting as consistent proteinuria (2), (20). Various phenotypes of liver and kidney diseases are associated with obesity and metabolic syndrome, including nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis, hepatocellular carcinoma, obesity-related glomerulopathy, urolithiasis, kidney cancer, and cardio-renal syndrome (2), (20). Among these, fatty liver disease is considered the predominant phenotype because 50%-80% of individuals with obesity also have NAFLD (21), compared to only 16% of individuals with normal BMI but lacking metabolic risk factors (22). Recently, the term MAFLD has been proposed to express the concept of liver diseases associated with known metabolic dysfunction (23), (24). AST and ALT levels were significantly higher in subjects who developed fatty liver disease than in those with non-fatty liver disease (25), and these levels thus provide significant biomarkers for the effect of weight loss interventions among individuals with NAFLD (6). Although obesity is not an essential precondition for proteinuria, weight gain concurrent with hypertension and glucose intolerance may be responsible for proteinuria as a proxy for kidney injury (26), (27). Based on this background, this study tested the impact of achieving weight loss and lifestyle modification on already developed metabolic-associated liver dysfunction (i.e., elevated levels of AST and/or ALT) and proteinuria. In our investigation, weight loss in the obese population appeared associated with better improvement in liver dysfunction (multivariable-adjusted HR 1.07, 95%CI 1.05-1.09 per −1 kg/m2/year), suggesting that metabolic-associated liver damage and kidney injury may be reversible among obese patients who are losing weight for whatever reason, interventional or non-interventional, consistent with previous studies in the context of NAFLD (6), (25).
Regarding the linkage between MAFLD and CKD, MAFLD offers a better identifier of patients with CKD than NAFLD, and MAFLD is strongly and independently associated with CKD and abnormal albuminuria (3). Moreover, one study demonstrated that individuals with fatty liver disease without metabolic syndrome were not at risk for the presence or incidence of CKD (5). This observation suggests the possibility of a shared mechanism between MAFLD and CKD, such as insulin resistance (28). Fatty liver diseases may exacerbate hepatic insulin resistance, promote hypertension, induce atherogenic dyslipidemia, and release a variety of pro-inflammatory molecules as pro-oxidant and pro-fibrogenic mediators that play important roles in the pathophysiology of CKD and other extrahepatic vascular complications (29), (30). A recent worldwide meta-analysis (30) identified only one study (31) examining the association between fatty liver associated with obesity and CKD in the Japanese population, so further research from Japan is sorely necessary.
In Japan, obesity is defined as a BMI of ≥25 kg/m2, and the presence of health problems requiring weight loss is also considered to be “obesity” (17). In Japan, few patients are severely obese with a BMI of >30 kg/m2, and although subjects receiving anti-obesity pharmacotherapy and weight-loss surgery are increasing, the numbers of individuals taking such steps remain relatively low and very few cases have been reported. Therefore, in reality, lifestyle interventions such as exercise and changes to dietary habits (32), (33) are likely to remain the mainstay of interventions for obese CKD patients in Japan for some time. Moreover, we clarified that the groups with a gain in exercise habits or quitting alcohol intake showed a significantly higher probability of improvement in liver dysfunction, but not improvements in proteinuria, than reference groups (Figure 3). Despite the fact that observational studies provide a lower level of evidence than randomized controlled trials, this result obtained from a large, general population cohort appears very useful in practice for obese populations or in providing health guidance after medical checkups in Japan. Although there is evidence that exercise does not increase urinary protein levels in CKD patients (34), (35), no studies have provided clear evidence that exercise reduces urinary protein. Similarly, concluding whether alcohol intake contributes to the amelioration of CKD presenting with urinary protein is also difficult based on current evidence. Instead, some evidence suggests that a small amount of alcohol in an individual with normal body weight (BMI 18.5-25 kg/m2) has suppressive effects on the development of CKD (36). Clear evidence is therefore currently lacking as to whether quitting alcohol intake is effective for reducing CKD and urinary protein. Nevertheless, this study had a mean observation period of only approximately 3 years, making it difficult to determine whether urinary protein is affected by the acquisition of exercise habits and quitting alcohol intake. We speculate that a longer observation period may reveal improvements in urinary protein with such lifestyle changes.
A key strength of this study was that to the best of our knowledge, it represents the first attempt to compare how body weight changes benefit Japanese individuals between those who have already developed liver dysfunction or proteinuria. However, some limitations must be kept in mind when interpreting the present findings. First, the implications of healthy weight loss as the main scope of the study, are very different when the fact of weight loss is due to complications and comorbidities. Second, since ultrasound results were not included in the specific health checkups, the diagnosis of fatty liver was only inferred from the coexistence of obesity and liver damage. Some research has pointed to liver dysfunction, obesity, and elevated ALT as significant predictors of NAFLD (37) and NAFLD is also diagnosed at a fairly high rate in liver disorders with obesity (22), (38). In this study, elevated liver enzymes in obese patients were considered as a surrogate marker for fatty liver. Third, γ-glutamyl transpeptidase is considered useful in the diagnosis of fatty liver as a marker of liver fibrosis but could not be included in this evaluation because of a lack of data during the follow-up period. Fourth, the relationship between weight change and eating habits was unclear due to a high frequency of missing data on eating habits, and clarification of the contribution of improved eating habits to weight change was not possible. Fifth, the obese population with liver dysfunction and/or proteinuria at baseline was so small that we were unable to elucidate whether weight changes and exercise habits independently affected improvement in liver dysfunction and proteinuria or instead affect these in relation to each other. Sixth, information regarding the use of drugs and lifestyle factors (i.e., smoking, exercise, and alcohol intake) were obtained via a self-reported questionnaire due to the design of the specific health checkups. Seventh, in the context of CKD, the incidence of adverse clinical outcomes differs significantly depending on the presence and severity of proteinuria. Because this is a cohort study utilizing a relatively healthy general population, the results are presented based on an analysis of persons with abnormal laboratory examinations regardless of the severity of proteinuria and renal dysfunction rather than primarily patients with advanced kidney disease who have a massive urinary protein. Eighth, since drug treatment for hypertension, hyperlipidemia, and diabetes may affect proteinuria, it would be interesting to track the number of years of treatment and the resulting changes in blood pressure, hyperlipidemia, and blood glucose after baseline, but this was not possible because of the design of the current study.
In conclusion, this observational cohort study suggested that weight loss equal to or greater than 1 kg/m2/year improves liver dysfunction and dipstick proteinuria in obesity. Particularly, liver dysfunction differs from urinary protein in that alleviation can be rapidly achieved with the acquisition of exercise habits and quitting alcohol intake.
None
This work was supported by Grants-in-Aid for “Research on Advanced Chronic Kidney Disease (REACH-J), Practical Research Project for Renal Disease” from the Japan Agency for Medical Research and Development (AMED) under grant numbers JP17ek0310005 and JP20ek0310010. This work was also supported by a Health and Labor Sciences Research Grant for “Study on the Design of the Comprehensive Health Care System for Chronic Kidney Disease (CKD) based on the individual risk assessment by Specific Health Check-Up” from the Ministry of Health, Labour and Welfare of Japan under grant number H24-nanchitou(jin)-ippan-006.
This study would not have been possible without the generous support of the public health nurses and the officials in each district. The authors would also like to thank Ikuko Takano and Ayumi Kaichi for their secretarial assistance.
Conceived and designed the study: KN and KY. Analyzed the data: KN. Collected the data: KN, KY, KA, and TW. Wrote the first draft of the manuscript: KN. Contributed to the writing and editing of the manuscript: TH, KM, KI, TM, KT, SF, IN, TK, MK, MK, YS, KA, TW, and KY. All authors agree with the manuscript results, conclusions, and publication.
The original ethics approval was obtained from Fukushima Medical University (approval nos. #1485 and #2771) and the institutional review board for ethical issues at the University of Tsukuba (approval no. 999; UMIN: 000019774). According to this approval, an official memorandum of understanding was exchanged between the institutional head and each mayor of the local government that owns the health checkup information from citizens. The protocol waives the need for individual consent because all data were obtained after concluding memoranda with the municipal heads. Information transfer was coordinated through local government officials, and only the output form without any individual data was disclosed to researchers. The names, addresses, and all other personalized data of participants were completely deleted from the linked data to protect privacy.
The data that support the findings of this study are available upon request to the last author (KY).
Matsuura B, Nunoi H, Miyake T, et al. Obesity and gastrointestinal liver disorders in Japan. J Gastroenterol Hepatol. 2013;28(Suppl 4):48-53.
Kovesdy CP, Furth SL, Zoccali C, et al. Obesity and kidney disease: hidden consequences of the epidemic. Kidney Int. 2017;91(2):260-2.
Sun DQ, Jin Y, Wang TY, et al. MAFLD and risk of CKD. Metabolism. 2021;115:154433.
Stenvinkel P, Zoccali C, Ikizler TA. Obesity in CKD--what should nephrologists know? J Am Soc Nephrol. 2013;24(11):1727-36.
Hashimoto Y, Hamaguchi M, Okamura T, et al. Metabolic associated fatty liver disease is a risk factor for chronic kidney disease. J Diabetes Investig. 2022;13(2):308-16.
Koutoukidis DA, Astbury NM, Tudor KE, et al. Association of weight loss interventions with changes in biomarkers of nonalcoholic fatty liver disease: a systematic review and meta-analysis. JAMA Intern Med. 2019;179(9):1303-4. Erratum for: JAMA Intern Med. 2019;179(9):1262-71.
Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2021;160(3):912-8.
Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121-9.
Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-e15.
Zhang HJ, He J, Pan LL, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern Med. 2016;176(8):1074-82.
Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57(1):157-66.
Sullivan S, Kirk EP, Mittendorfer B, et al. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55(6):1738-45.
Navaneethan SD, Yehnert H, Moustarah F, et al. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4(10):1565-74.
Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant. 2013;28(Suppl 4):iv82-iv98.
Nagai K, Yamagata K, Iseki K, et al. Weight loss reduces the incidence of dipstick proteinuria: a cohort study from the Japanese general population. Clin Exp Nephrol. 2021;25(12):1329-35.
World Health Organization. Obesity and overweight 2021 [Internet]. [cited 2022 Sep 30]. Available from: https://www.who.int/westernpacific/health-topics/obesity#:~:text=It%20is%20defined%20as%20a,equal%20to%2030%20is%20obesity.
Japan Society for the Study of Obesity (JASSO). Definition of obesity [Internet]. 2021 [cited 2022 Sep 30]. Available from: http://www.jasso.or.jp/data/magazine/pdf/chart_A.pdf. Japanese.
Nagai K, Yamagata K, Iseki K, et al. Cause-specific mortality in the general population with transient dipstick-proteinuria. PLoS One. 2019;14(10):e0223005.
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):746. Erratum for: Lancet. 2014;384(9945):766-81.
Matsuura B, Nunoi H, Miyake T, et al. Obesity and gastrointestinal liver disorders in Japan. J Gastroenterol Hepatol. 2013;28(Suppl 4):48-53.
Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82-97.
Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124-31.
Eslam M, Sanyal AJ, George J, et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999-2014.e1.
Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202-9.
Yoshioka N, Ishigami M, Watanabe Y, et al. Effect of weight change and lifestyle modifications on the development or remission of nonalcoholic fatty liver disease: sex-specific analysis. Sci Rep. 2020;10(1):481.
Obermayr RP, Temml C, Knechtelsdorfer M, et al. Predictors of new-onset decline in kidney function in a general middle-European population. Nephrol Dial Transplant. 2008;23(4):1265-73.
Othman M, Kawar B, El Nahas AM. Influence of obesity on progression of non-diabetic chronic kidney disease: a retrospective cohort study. Nephron Clin Pract. 2009;113(1):c16-23.
Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 2021;6(7):578-88.
Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. 2014;64(4):638-52.
Mantovani A, Zaza G, Byrne CD, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: a systematic review and meta-analysis. Metabolism. 2018;79:64-76.
Arase Y, Suzuki F, Kobayashi M, et al. The development of chronic kidney disease in Japanese patients with non-alcoholic fatty liver disease. Intern Med. 2011;50(10):1081-7.
Howden EJ, Leano R, Petchey W, et al. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol. 2013;8(9):1494-501.
Ikizler TA, Robinson-Cohen C, Ellis C, et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol. 2018;29(1):250-9.
Headley S, Germain M, Milch C, et al. Exercise training improves HR responses and V˙O2peak in predialysis kidney patients. Med Sci Sports Exerc. 2012;44(12):2392-9.
Howden EJ, Coombes JS, Strand H, et al. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis. 2015;65(4):583-91.
Yamagata K, Ishida K, Sairenchi T, et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 2007;71(2):159-66.
Abd El-Wahab EW, Zein El-Abedin RA, Ahmed WM, et al. Validation of a non-laboratory based screening tool for predicting non-alcoholic fatty liver disease in an Egyptian setting. Am J Med Sci. 2020;360(6):662-77.
Hashem A, Khalouf A, Acosta A. Management of obesity and nonalcoholic fatty liver disease: a literature review. Semin Liver Dis. 2021;41(4):435-47.