Corresponding author: Takashi Nakamura, takashinak830@gmail.com
DOI: 10.31662/jmaj.2024-0201
Received: July 30, 2024
Accepted: September 9, 2024
Advance Publication: November 11, 2024
Published: January 15, 2025
Cite this article as:
Nakamura T, Fujikawa H, Uenishi N. Brain Magnetic Resonance Imaging Findings of Shiga Toxin-producing Escherichia coli Hemolytic Uremic Syndrome-associated Encephalopathy. JMA J. 2025;8(1):298-299.
Key words: Shiga toxin-producing Escherichia coli, hemolytic uremic syndrome, encephalopathy, neurological manifestation, seizure, head magnetic resonance imaging, thin-section, pons
An 18-year-old female presented to our hospital with a 3-day history of hematochezia. Laboratory examination revealed hemolytic anemia, thrombocytopenia, and acute kidney injury. Stool cultures of Shiga toxin-producing Escherichia coli (STEC) O157: H7 confirmed the presence of typical hemolytic uremic syndrome (HUS).
Postadmission, she developed generalized tonic-clonic seizures. Brain magnetic resonance imaging (MRI) revealed bilateral symmetrical T2-fluid-attenuated inversion recovery (FLAIR) hyperintensities in the dorsal pontine base and thalamus (Figure 1A and 1B). The lesions showed high signal at diffusion-weighted imaging and apparent diffusion coefficient maps, indicating T2-shine through (Figure 1C, 1D, 1E and 1F). Considering the clinical course and imaging findings, this case was diagnosed with STEC-HUS-associated encephalopathy. After five rounds of plasma exchange and levetiracetam administration, she remained seizure-free and MRI signal changes improved (Figure 1G, 1H, 1I, 1J, 1K and 1L).
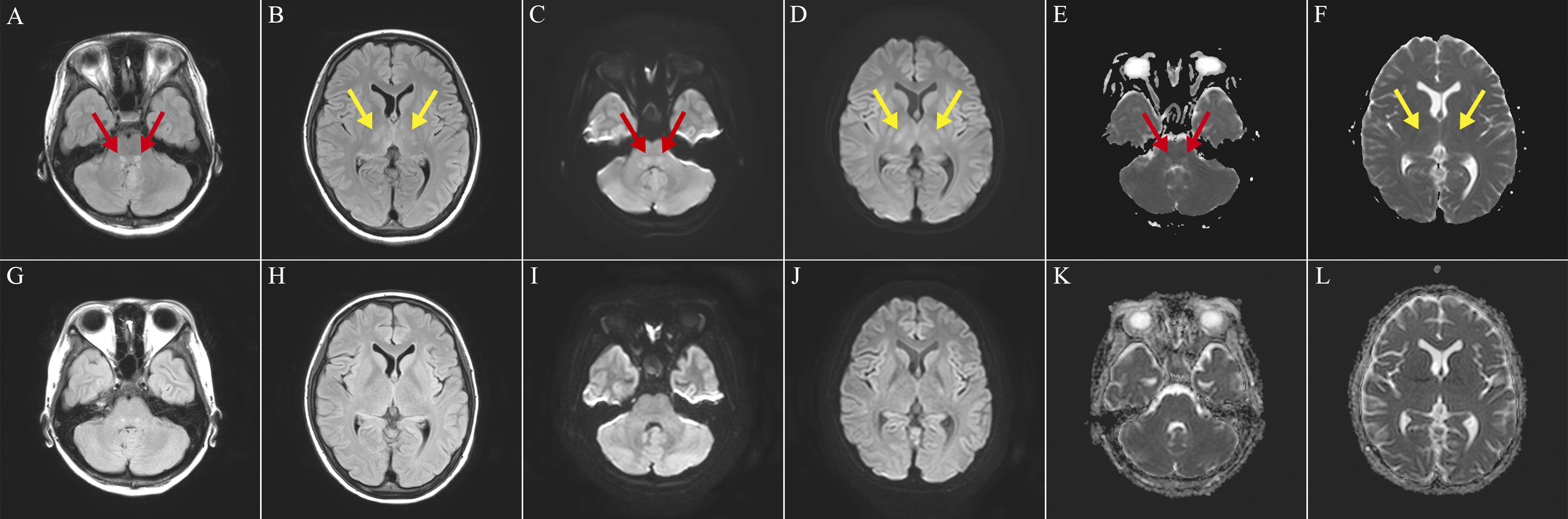
Neurological manifestations of STEC-HUS are life-threatening complications, with an incidence of 17%-34%, and fatal prognostic factors (1), (2). Upon imaging, the lesions imply a nearly symmetrical distribution pattern, including bilateral basal ganglia, bilateral centrum semiovale, bilateral thalami, and bilateral brainstem (3). Among them, transient symmetric vasogenic edema of the central pons (T2-weighted or T2-FLAIR imaging) is a relatively characteristic finding (4). Transient T2 hyperintensities in the brainstem have been observed in patients with other diseases, including metronidazole encephalopathy and herpes simplex virus encephalitis (4). However, in this case, because the diagnosis of STEC-HUS was bacteriologically confirmed and no history of other diseases (e.g., no metronidazole use and no fever to support the diagnosis of herpes simplex virus encephalitis) was suggested, STEC-HUS-associated encephalopathy was diagnosed. Pontine hyperintensities are typically small and can be overlooked. Thin-section (3 mm) brain MRI may lead to an accurate diagnosis, thereby improving prognosis (4).
None
TN acquired data and drafted the manuscript. HF and NU reviewed and supervised the manuscript.
Informed consent was obtained for this manuscript.
IRB approval was not required for this study.
Alconcher LF, Coccia PA, Suarez ADC, et al. Hyponatremia: a new predictor of mortality in patients with Shiga toxin-producing Escherichia coli hemolytic uremic syndrome. Pediatr Nephrol. 2018;33(10):1791-8.
Khalid M, Andreoli S. Extrarenal manifestations of the hemolytic uremic syndrome associated with Shiga toxin-producing Escherichia coli (STEC HUS). Pediatr Nephrol. 2019;34(12):2495-507.
Mansour MA, Khalil DF, Hasham MA, et al. Hemolytic uremic syndrome with central nervous system manifestations, a case report and literature review. Radiol Case Rep. 2023;18(6):2268-73.
Wengenroth M, Hoeltje J, Repenthin J, et al. Central nervous system involvement in adults with epidemic hemolytic uremic syndrome. AJNR Am J Neuroradiol. 2013;34(5):1016-21.