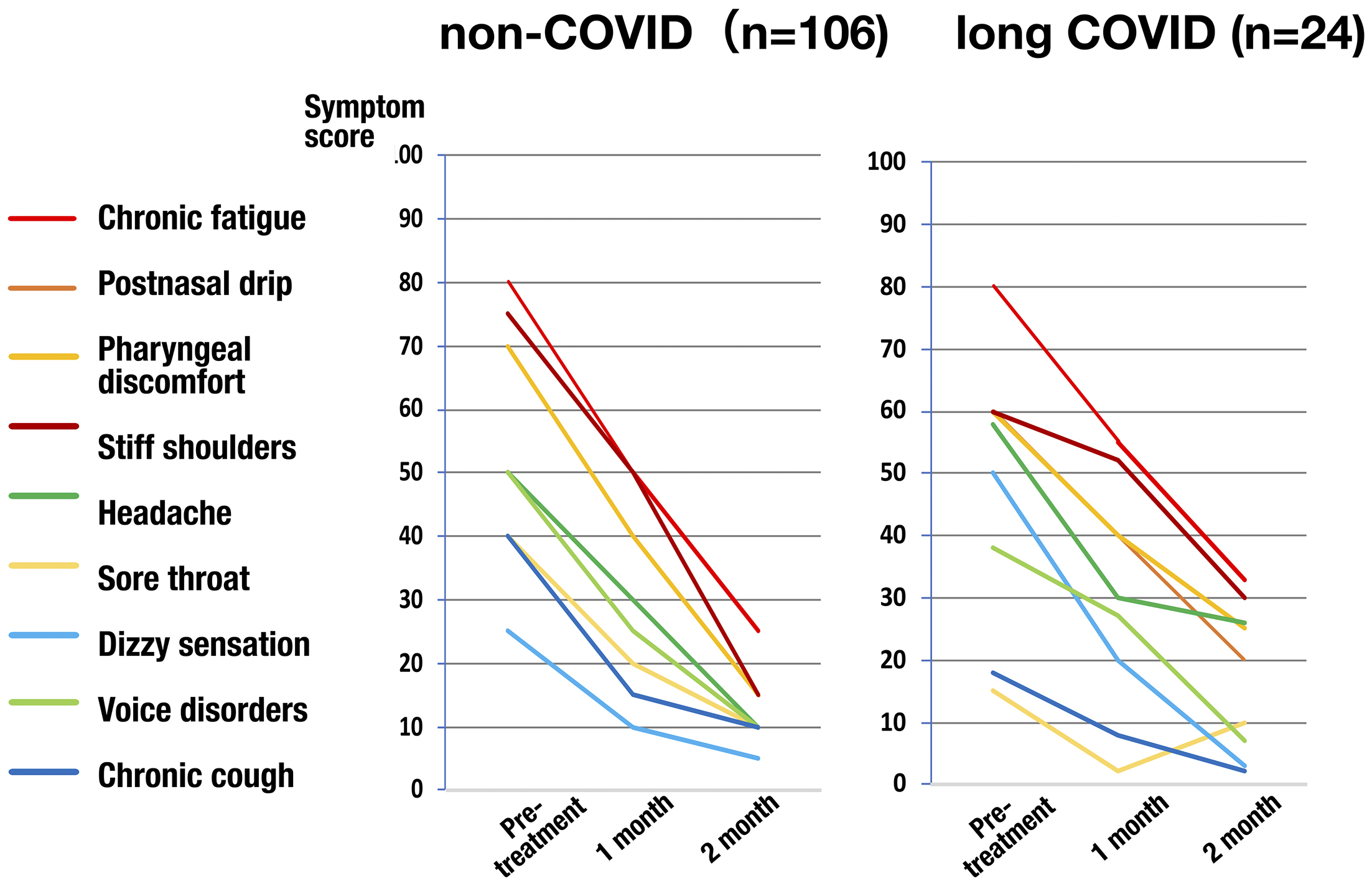
Figure 1. Scoring for the severity of epipharyngeal inflammation. The severity of four epipharyngeal endoscopic findings, (1) redness, (2) swelling of the mucous membrane, (3) mucus adhesion/postnasal drip, and (4) bleeding due to abrasion, be scored on a three-point scale from 0 to 2, respectively, and the sum of these scores be used as an index of epipharyngeal inflammation.
From: Retracted: Chronic Epipharyngitis Treated with Epipharyngeal Abrasion Therapy: Symptoms, Diagnosis, Pathogenesis, and Treatment Outcomes
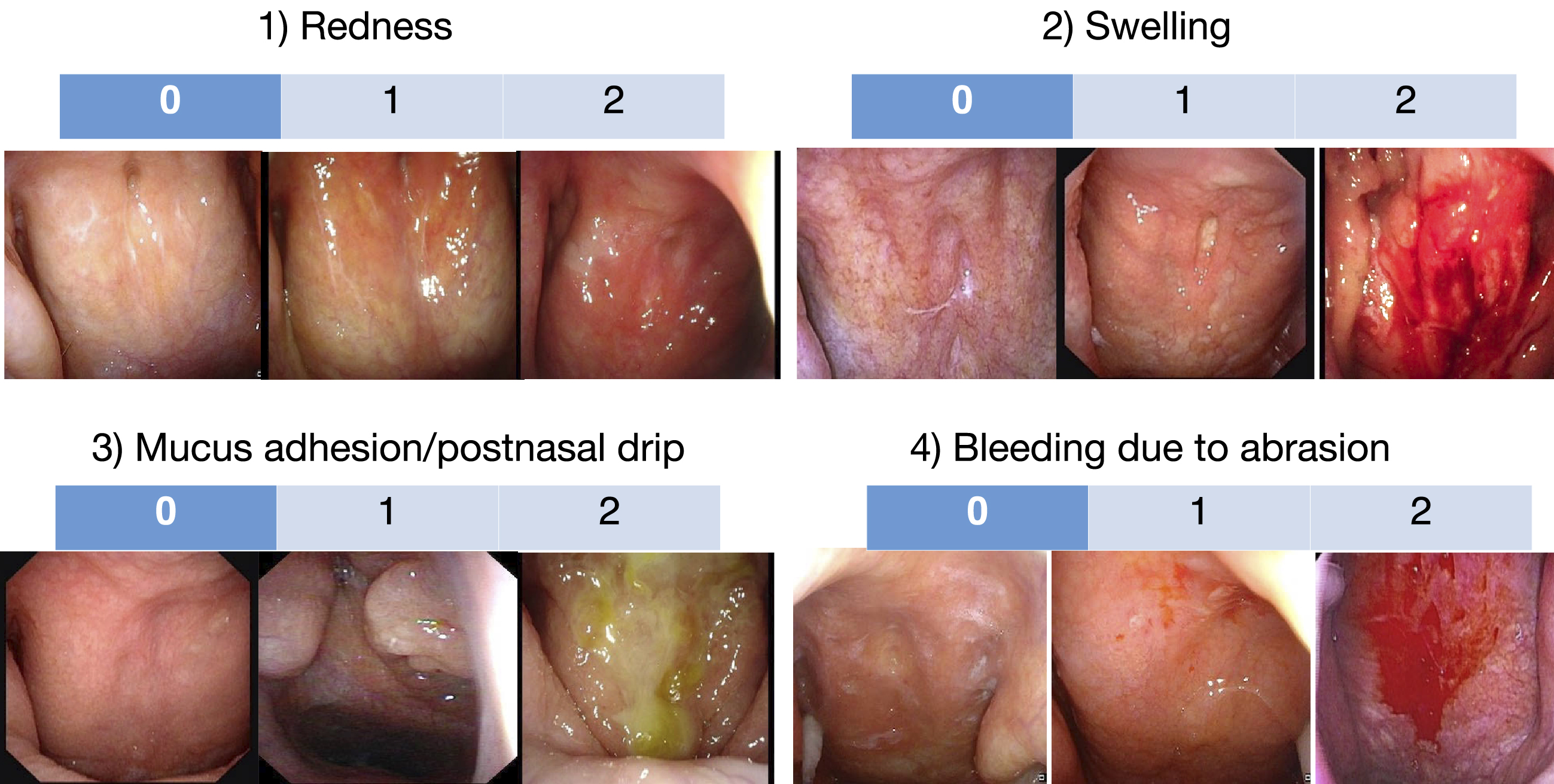
Figure 2. Symptoms and pathogenesis of chronic epipharyngitis. The epipharyngeal lymphoid tissue, including the pharyngeal tonsils, which are located at the entrance to the upper respiratory tract and have a primary immune response to various antigens, is stimulated by innate immune ligands such as viral RNA, bacterial DNA as well as immunostimulatory adjuvants such as aluminum contained in HPV vaccines and SARS-CoV-2 vaccines, causing chronic inflammation and edema in the epipharyngeal tissue through the release of inflammatory cytokines. As a result, (1) local symptoms due to inflammation of the epipharynx, (2) functional somatic symptoms mediated by dysfunction of the hypothalamus-limbic system due to disturbances of vagal response and cerebrospinal fluid outflow, and (3) distant organ symptoms caused by the epipharynx lymphatic tissue as an etiologic organ.
DNA: deoxyribonucleic acid; HPV: human papillomavirus; RNA: ribonucleic acid; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
From: Retracted: Chronic Epipharyngitis Treated with Epipharyngeal Abrasion Therapy: Symptoms, Diagnosis, Pathogenesis, and Treatment Outcomes
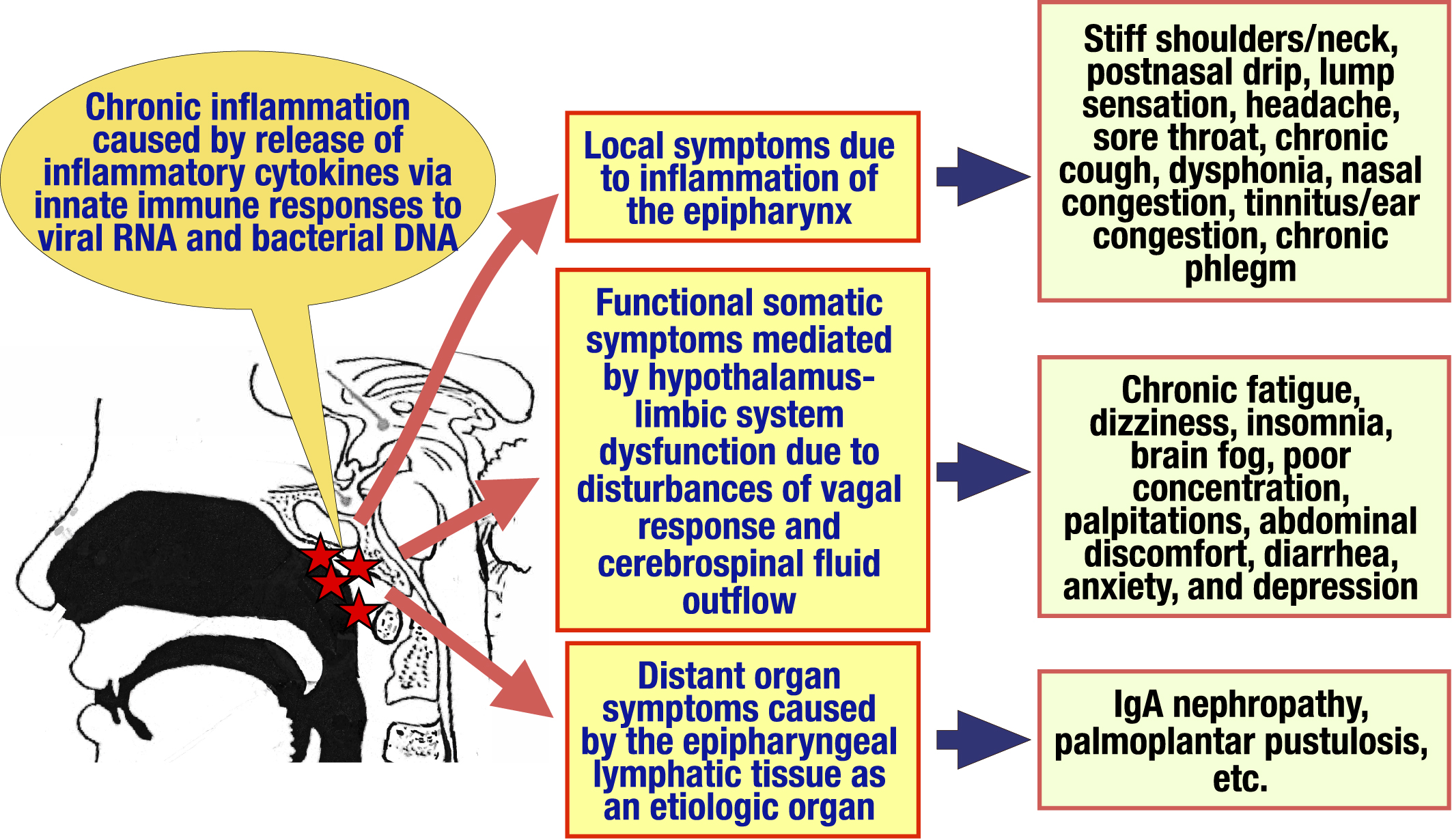
Figure 3. Impaired vagal response in chronic epipharyngitis. The vagal response is known to have anti-inflammatory effects through the cholinergic anti-inflammatory pathway and the STN- PVN-pituitary-adrenal pathway, and prevents potentially harmful inflammation. Tactile and visceral sensations in the epipharynx stimulate vagal afferent pathways, which activate the STN in the medulla oblongata. The signal is transmitted to the DMN via PVN in the hypothalamus and to the efferent vagus nerve pathway. The pathway further branches through the nodose ganglion to various organs such as the digestive tract, pancreas, and spleen, and inflammation is suppressed by the release of neurotransmitters such as acetylcholine at the terminals of each organ. In another pathway, the signals transmitted to the vagus afferent tract-STN-PVN activate the pituitary gland, ultimately resulting in the secretion of cortisol from the adrenal gland. Furthermore, activation signals transmitted from the STN to the PVN activate the hypothalamus-limbic system via the area postrema, regulating emotion, cognition, and behavior. In chronic epipharyngitis, on the other hand, inflammatory cytokines such as IL-1, IL-6, and TNFα, which are continuously overproduced by chronic inflammation, overstimulate the vagal afferent tract. The resulting vagal response is impaired, leading to inflammation in various organs, decreased cortisol secretion, and hypothalamus-limbic system dysfunction, followed by multi-organ symptoms as well as functional somatic symptoms.
DMN: dorsal motor nucleus; IL: interleukin; PVN: paraventricular nucleus; STN: solitary tract nucleus; TNFα: tumor necrosis factor-α.
From: Retracted: Chronic Epipharyngitis Treated with Epipharyngeal Abrasion Therapy: Symptoms, Diagnosis, Pathogenesis, and Treatment Outcomes
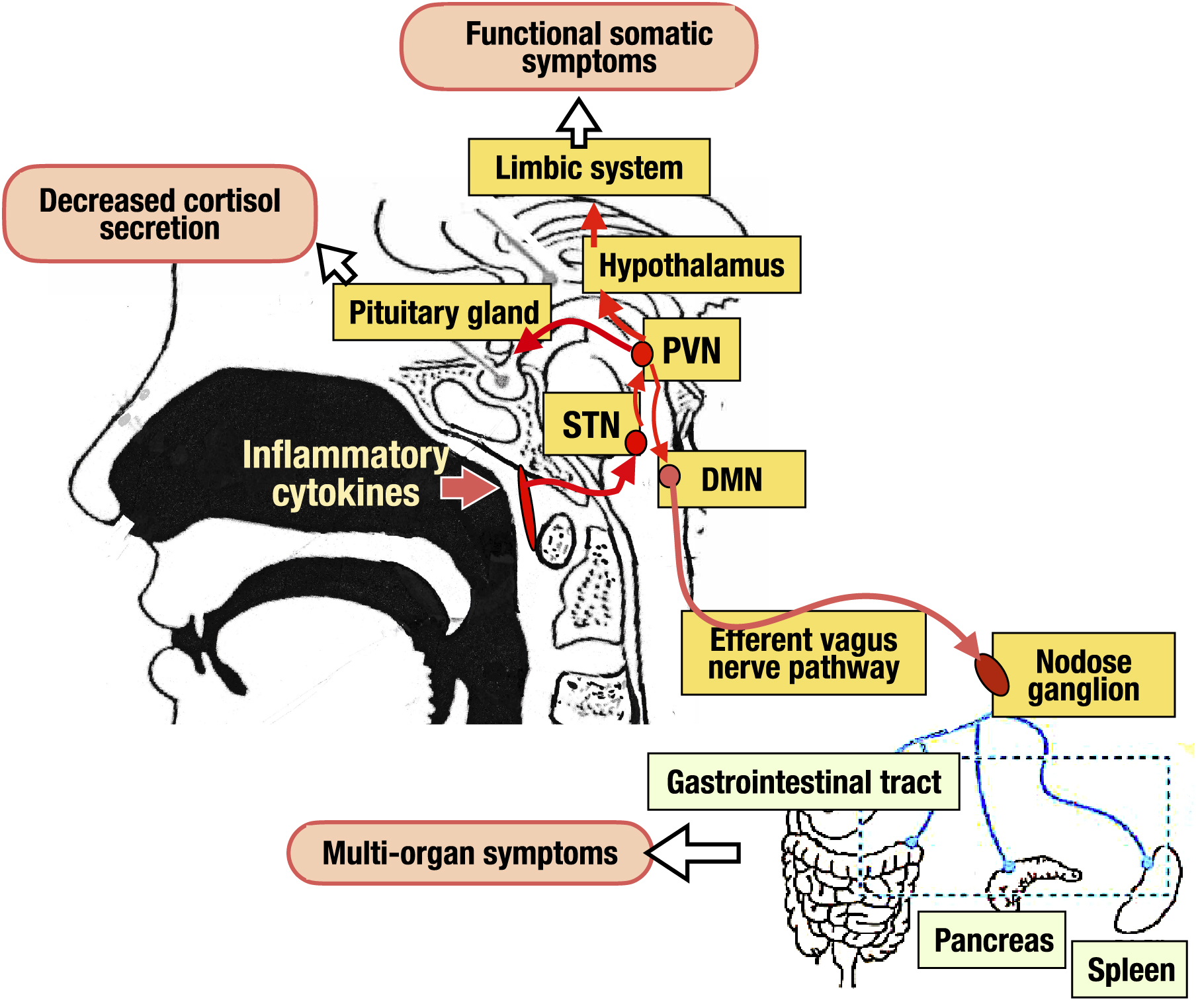
Figure 4. Pathogenesis of IgA nephropathy and palmoplantar pustulosis involving epipharyngeal lymphoid tissue as an etiologic organ. IgA nephropathy and palmoplantar pustulosis are categorized as TIAS. Patients with TIAS have a breakdown of immune tolerance to tonsillar resident bacteria, resulting in a hyperimmune response to these microorganisms. As a result, tonsillar B-cells are activated, and autoantibodies to keratin and HSPs, which are common antigens to the skin are produced in patients with palmoplantar pustulosis. In patients with IgA nephropathy, mutant IgA is produced through the B cell-activating factor (BAFF)/proliferation-inducing ligand (APRIL)-mediated pathway. Such substances are transported to the skin and renal glomeruli through the systemic circulation, and then deposited in these tissues. In addition, tonsillar T-cells expressing CLA or chemokine receptors (CCR6, CXCR3, CX3CR1), which have an affinity for skin or renal glomeruli, are activated, home to the skin and renal glomeruli through the systemic circulation, and then, tissue damage occurs in the target organs. The epipharyngeal lymphoid tissue has the same immune response mechanism as the palatine tonsil. Therefore, the epipharyngeal lymphoid tissue of patients with IgA nephropathy and palmoplantar pustulosis is the etiologic organ of these diseases, as is the palatine tonsil.
CLA: cutaneous lymphocyte-associated antigen; HSP: heat shock protein; IgA: immunoglobulin A; TIAS: tonsil-induced autoimmune/inflammatory syndrome.
From: Retracted: Chronic Epipharyngitis Treated with Epipharyngeal Abrasion Therapy: Symptoms, Diagnosis, Pathogenesis, and Treatment Outcomes
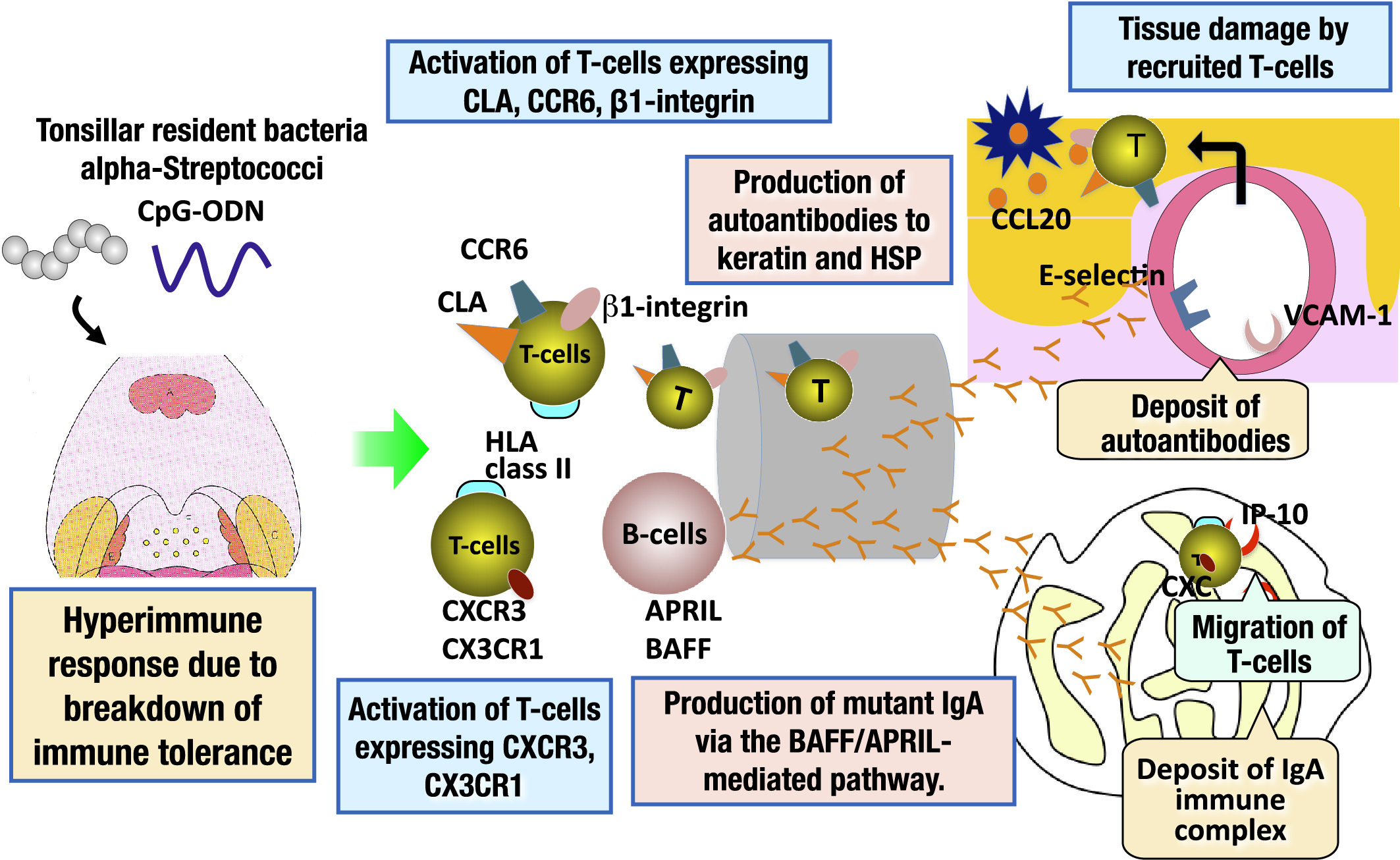
Figure 5. The technique of transnasal E-EAT. A) First, tap the center of the posterior wall of the epipharynx lightly 5 to 6 times with a slightly curved nasal cotton applicator to check for bleeding or edema of the mucosa. Next, abrade the mucosa widely from the midline of the epipharynx to the left and right, and from the top to the bottom of the posterior wall, while pointing the applicator up and down. The basic principle is to “abrasively” the mucosa with enough strength to lightly abrade it, rather than apply. If there is inflammation, the edematous mucosa is likely to bleed and mucosal peeling will be seen. B) Then, abrade the left and right Rosenmüller’s fossa with a slightly curved nasal cotton applicator facing downward. C) Finally, abrade the dorsal side of the soft palate toward the front with a slightly curved nasal cotton applicator facing downward. D) The left and right ends of the roof of the epipharynx and the Rosenmüller’s fossa are likely to be insufficiently abraded with a slightly curved nasal cotton applicator. In that case, it is easier to reach them by using a nasal cotton applicator that is more strongly curved. Abrade the right epipharynx roof with the strongly curved nasal cotton applicator, then slide it into the right Rosenmüller’s fossa and move the applicator in a rotary or wiper-like motion. If the internal carotid artery is close to the Rosenmüller fossa, mucosal pulsation may be seen. Caution is advised.
E-EAT: endoscopic epipharyngeal abrasive therapy.
From: Retracted: Chronic Epipharyngitis Treated with Epipharyngeal Abrasion Therapy: Symptoms, Diagnosis, Pathogenesis, and Treatment Outcomes
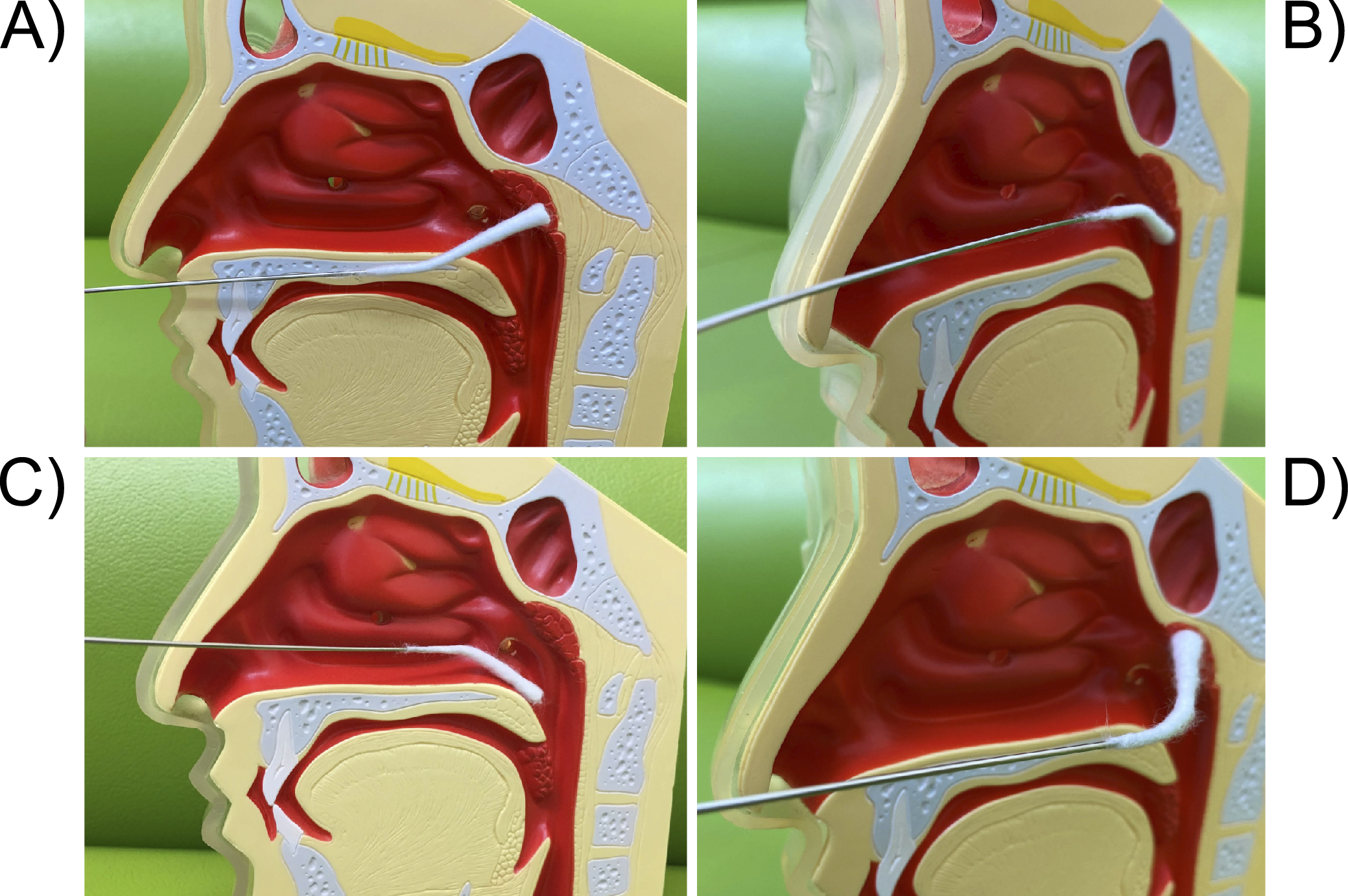
Figure 6. Technique for transoral E-EAT. A) Since the tip of the pharyngeal cotton applicator cannot be clearly seen when inserted into the oral cavity, check the low position of the uvula, the width of the palatine ischium, and the degree of the pharyngeal reflex in advance. Gently press the dorsum of the tongue with a tongue depressor, insert the pharyngeal cotton applicator horizontally into the oral cavity, and flick it upwards when it hits the posterior wall of the oropharynx. For the posterior wall of epipharynx, move the pharyngeal cotton applicator back and forth like a wiper, rubbing it toward you. Abrade the entire surface including the epipharyngeal roof and lateral walls. When abrading the canopy, be careful not to damage it by not using the mandibular teeth as a fulcrum. B) Also, curving the pharyngeal cotton applicator more strongly will make it easier to reach the canopy.
E-EAT: endoscopic epipharyngeal abrasive therapy.
From: Retracted: Chronic Epipharyngitis Treated with Epipharyngeal Abrasion Therapy: Symptoms, Diagnosis, Pathogenesis, and Treatment Outcomes
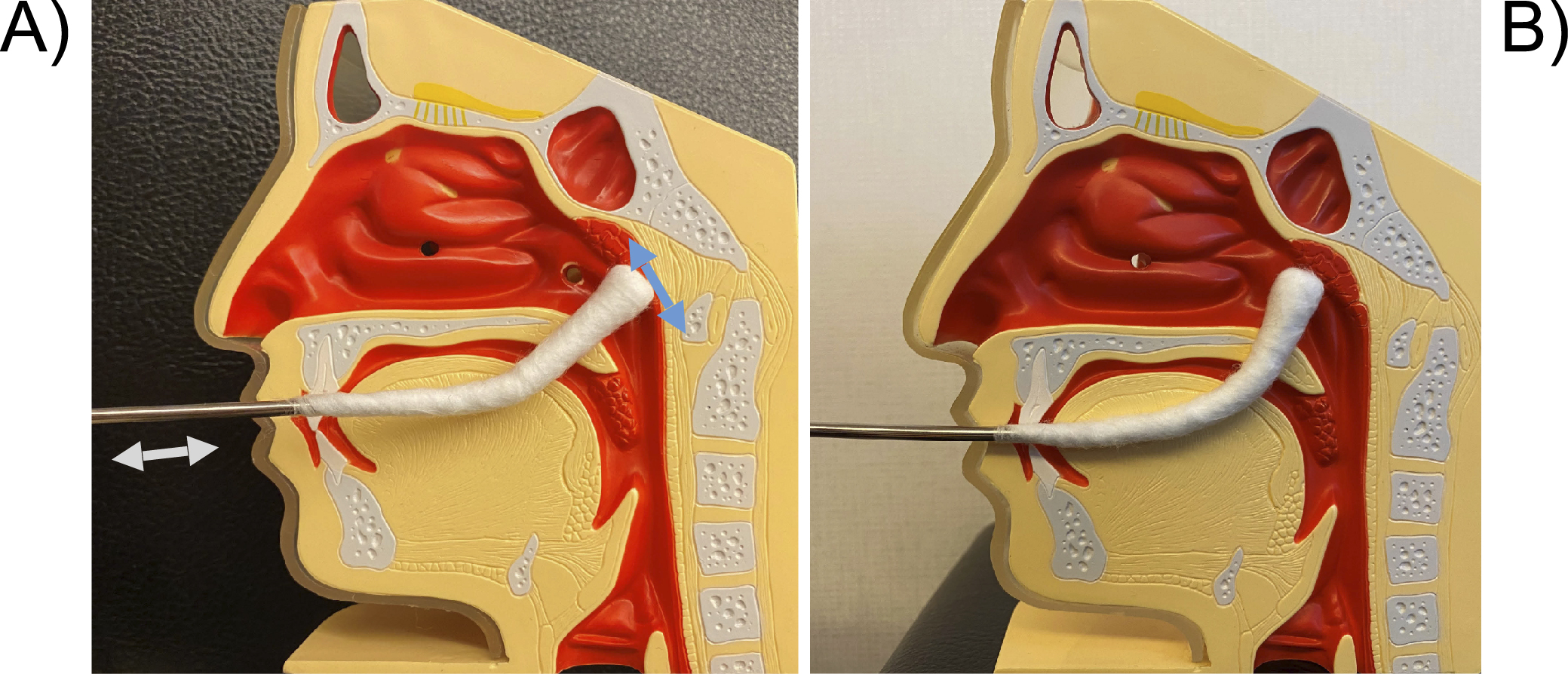
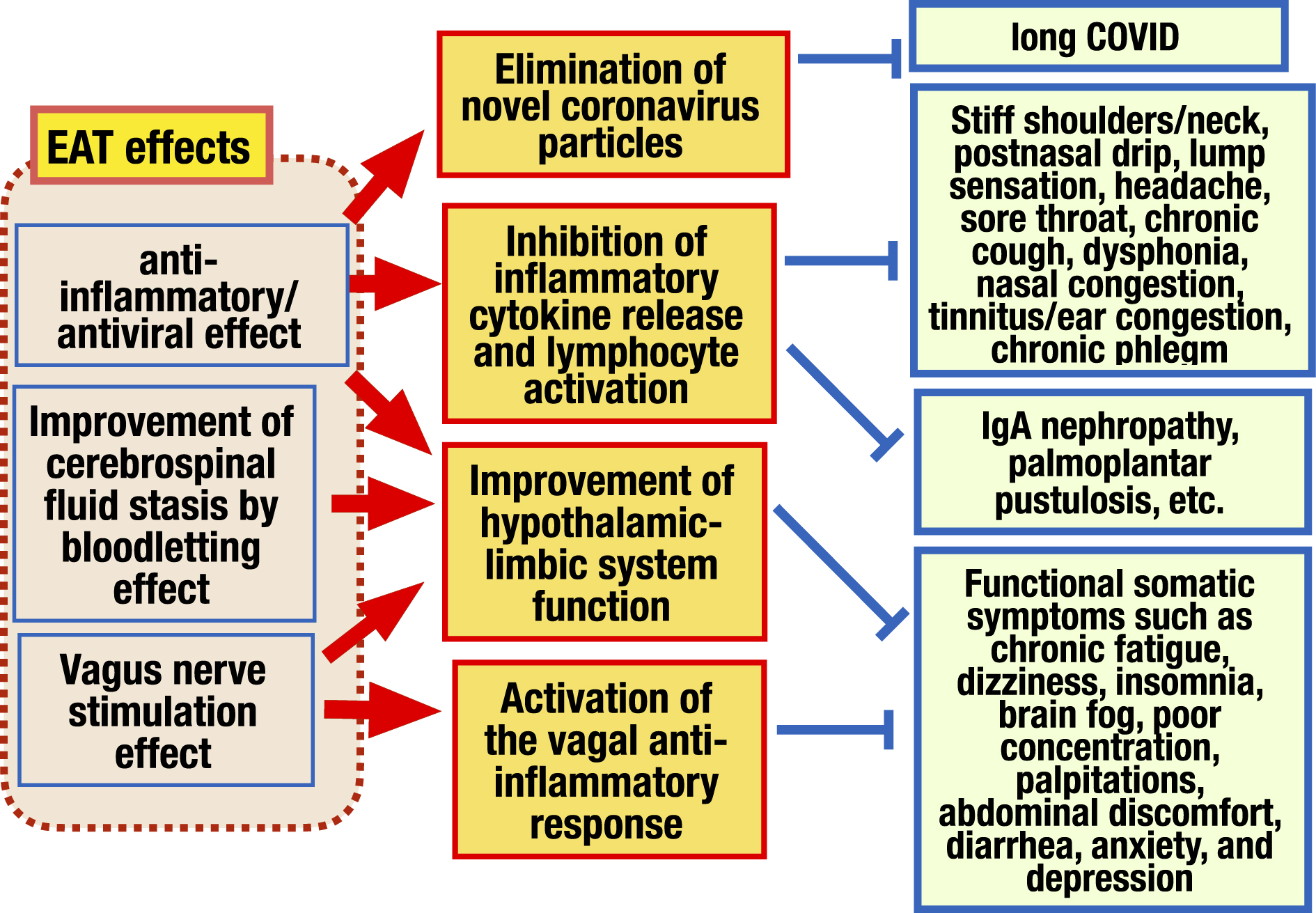
Figure 7. Mechanism of EAT effects. EAT effects are broadly composed of 3 categories, 1) anti-inflammatory/antiviral effect, 2) bloodletting effect, and 3) vagus nerve stimulating effect. 1) EAT has an anti-inflammatory effect. It suppresses the release of inflammatory cytokines and the activation of lymphocytes in the epipharyngeal lymphoid tissue, improving direct symptoms caused by inflammation of the epipharynx. It also reduces edema in the epipharynx, improves cerebrospinal fluid outflow disorders and vagus nerve response disorders, and then, recovers from various functional somatic symptoms caused by hypothalamic-limbic dysfunction. EAT has antiviral effects also and improves long COVID. 2) When EAT is performed, blood and interstitial fluid leakage is observed from the epipharyngeal mucosa. This is a bloodletting effect that mechanically removes interstitial fluid, lymphocytes, and inflammatory cytokines stagnated under the mucosa. The bloodletting effect improves cerebrospinal fluid outflow disorders caused by lymphatic congestion and restores hypothalamic to limbic system function. 3) EAT stimulates vagal afferent fibers abundant in the epipharynx, activating the nucleus of the STN located in the medulla oblongata several centimeters behind the epipharynx, resulting in inducing the anti-inflammatory effect and the hypothalamic-limbic regulatory effect, of the vagal response.
EAT: epipharyngeal abrasive therapy; long COVID: post-acute sequelae of COVID-19 STN: solitary tract nucleus.
From: Retracted: Chronic Epipharyngitis Treated with Epipharyngeal Abrasion Therapy: Symptoms, Diagnosis, Pathogenesis, and Treatment Outcomes