Corresponding author: Shunichi Sugawara, swara357@sendai-kousei-hospital.jp
DOI: 10.31662/jmaj.2019-0015
Received: March 11, 2019
Accepted: November 7, 2019
Advance Publication: December 27, 2019
Published: January 15, 2020
Cite this article as:
Tsurumi K, Kawashima Y, Akahira J, Saito R, Toi Y, Nakamura A, Yamanda S, Kimura Y, Honda Y, Sugawara S. A Remarkable Clinical Response to Pembrolizumab in a Rare Spindle Cell Carcinoma of the Lung. JMA J. 2020;3(1):83-86.
Spindle cell carcinoma of the lung consists of only spindle-shaped tumor cells, and accounts for approximately 13.3% of all sarcomatoid carcinomas (SCs), which are a rare subtype of poorly differentiated non-small cell lung cancer (NSCLC). Spindle cell carcinoma of the lung has very poor prognosis owing to resistance to chemotherapy and radiotherapy. This case report describes a 76-year-old man who presented with complaints of dry cough and right-sided neck pain and was later diagnosed with spindle cell carcinoma of the lung. He had a medical history of type 2 diabetes, angina pectoris, atrial fibrillation, hypertension, hyperlipidemia, and hepatitis B and a 20 pack-year history of smoking. A computed tomography (CT) scan revealed a mass with a thick-walled cavity in the right upper lobe of the lung. His neck pain was consistent with PET-CT images, indicating metastases due to invasion of lung cancer cells. The expression of PD-L1 in more than 90% of the tumor cells of the lung biopsy tissue led to the administration of pembrolizumab. The lung and metastatic tumors dramatically decreased in size after 9 weeks, and no tumor regrowth was observed over 11 courses of pembrolizumab administration. To the best of our knowledge, there are no previous reports describing the use of pembrolizumab for spindle cell carcinoma of the lung. This case report suggests that immunotherapy could be a promising treatment option for rare types of lung cancers, including spindle cell carcinoma.
Key words: Spindle cell carcinoma, Pembrolizumab, PD-L1, Sarcomatoid carcinoma
Sarcomatoid carcinoma (SC) is a rare subtype of poorly differentiated non-small cell lung cancer (NSCLC), accounting for 0.1%–0.4% of all malignant tumors of the lung (1). According to the latest World Health Organization (WHO) classification, SC comprises of five histopathological subtypes, namely, pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma. Spindle cell carcinoma consists of only spindle-shaped tumor cells and accounts for approximately 13.3% of all SCs (2). Spindle cell carcinoma of the lung often affects older male smokers, and the prognosis is very poor due to resistance to chemotherapy and radiotherapy (3). To the best of our knowledge, we are the first to report a case of spindle cell carcinoma of the lung treated with pembrolizumab.
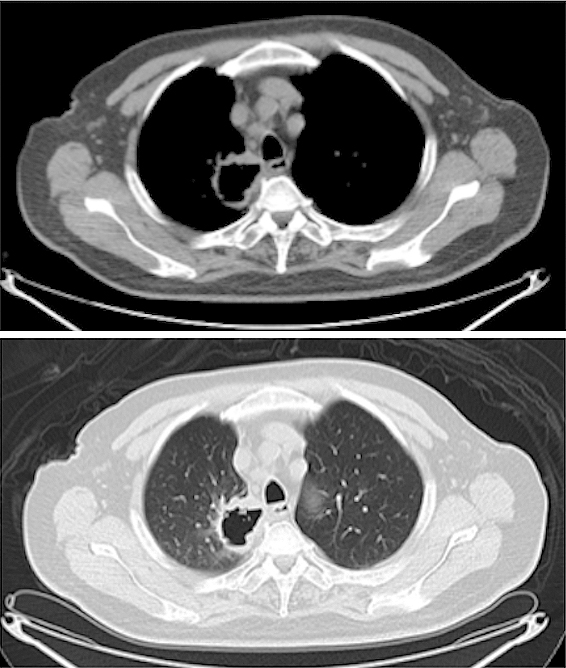
A 76-year-old man visited the hospital complaining of dry cough and right-sided neck pain. He had a past medical history of type 2 diabetes, angina pectoris, atrial fibrillation, hypertension, hyperlipidemia, and hepatitis B and a 20 pack-year history of smoking. A computed tomography (CT) scan showed a mass with a thick-walled cavity in the right upper lobe of the lung (Figure 1). A transbronchial lung biopsy revealed proliferation of spindle cells which were immunohistochemically positive for cytokeratin and vimentin.
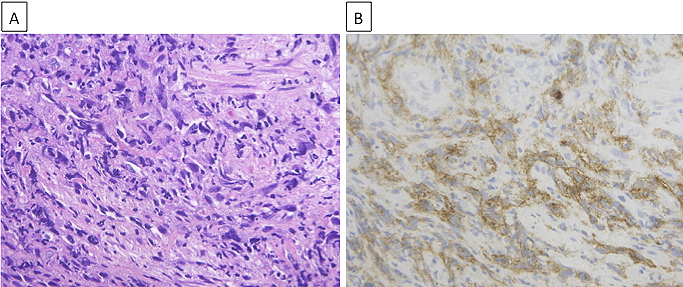
Although the limited size of the biopsy specimen made a thorough analysis difficult, we confirmed spindle cells by hematoxylin and eosin (H&E) staining (Figure 2A), which aided in diagnosing the patient with spindle cell carcinoma of the lung. While gene mutation test results for epidermal growth factor receptor mutations and anaplastic lymphoma kinase rearrangement were negative, programmed death ligand-1 (PD-L1) expression was observed in more than 90% of the tumor cells of the lung biopsy tissue (Figure 2B) by immunohistochemistry (IHC) using the PD-L1 IHC 22C3 pharmDx antibody (clone 22C3; Dako North America Inc.). A positron emission tomography (PET)-CT scan showed fluorodeoxyglucose uptake in the mediastinal and hilar lymph nodes, right adrenal gland, and right trapezius muscle, which was consistent with his symptom (Figure 3A). Based on all these findings, the patient was diagnosed with advanced lung cancer, spindle cell carcinoma, cT4N2M1c, stage IVB.
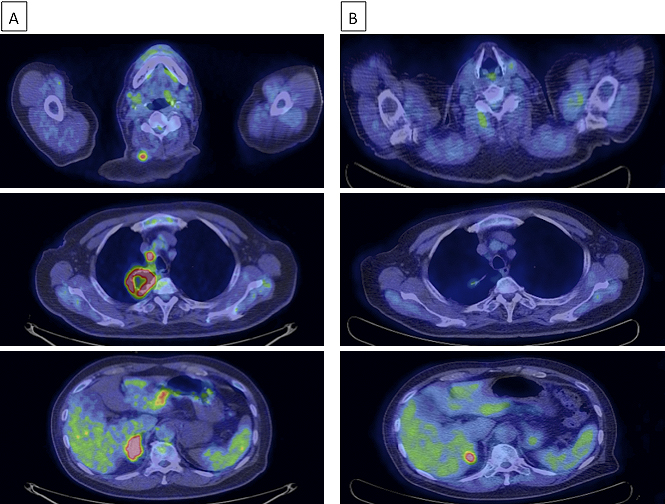
From April 2017, the patient began receiving 200 mg pembrolizumab intravenously every 3 weeks. The lung and metastatic tumors dramatically decreased in size after 9 weeks, and tumor regression was maintained after 21 weeks, as indicated by the reduced fluorodeoxyglucose uptake observed in the PET-CT scan (Figure 3B). The tumor continued to reduce in size, and the patient’s complaint of neck pain was resolved.
Recently, patients with advanced NSCLC have demonstrated significantly longer overall survival after treatment with immune checkpoint inhibitors than those after standard chemotherapy. PD-L1 is a promising biomarker for immunotherapy. Multiple clinical trials suggest that PD-L1 expression in tumors predicts response to immune checkpoint inhibitors(4), (5), (6), (7).
High PD-L1 expression in SC has been previously reported(8), where subjects with SC were predominantly men (10 of 13) with an average age of 60.2 years. Of the 13 SC patients (including 1 spindle cell carcinoma), 9 (69.2%) were positive for PD-L1 expression, indicating a potential use for immune checkpoint inhibitors in SC.
While there have been some case reports on the dramatic and long-lasting efficacy of immune checkpoint inhibitors in pulmonary pleomorphic carcinoma, the most prevalent type of SC(9), (10), there are no previous reports describing the use of pembrolizumab for spindle cell carcinoma of the lung. In this case, the limited specimen obtained from the transbronchial lung biopsy made the diagnosis challenging, and we relied on our pathologist’s judgment who diagnosed the patient with spindle cell carcinoma. While we acknowledge this diagnostic ambivalence, and the need for further investigation, our clinical results suggest that immunotherapy could be a promising treatment option for rare forms of lung cancer, including spindle cell carcinoma of the lung.
Shunichi Sugawara received honorariums from AstraZeneca, Chugai Pharma, Pfizer, Taiho Pharmaceutical, Eli Lilly and Company, Novartis, Kyowa Hakko Kirin, Bristol-Myers Squibb, Ono Pharmaceutical, MSD K.K, Nippon Boehringer Ingelheim; Atsushi Nakamura received honorarium from MSD K.K; Yukihiro Toi received honorarium from MSD K.K.
We thank Editage for their English editorial assistance.
Kyoji Tsurumi, Yosuke Kawashima, Ryohei Saito and Shunichi Sugawara managed the patient. Junichi Akahira evaluated the pathology slides. Yukihiro Toi, Atsushi Nakamura, Shinsuke Yamanda, Yuichiro Kimura and Yoshihiro Honda assisted preparation of the manuscript. All authors contributed to writing the report. Written consent for publication was obtained.
This manuscript is a case report and does not need approval by IRB.
Written informed consent was obtained from the patient publishing this case report and accompanying images.
Travis WD, Brambilla E, Burke AP, et al. WHO Classification of tumors of the lung, pleura, thymus and heart. 4th ed. IARC; 2015. p. 88-94.
Rossi G, Cavazza A, Sturm N, et al. Pulmonary carcinomas with pleomorophic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol. 2003;27(3):311-24.
Mainwaring MG, Poor C, Zander DS, et al. Complete remission of pulmonary spindle cell carcinoma after treatment with oral germanium sesquioxide. Chest. 2000;117(2):591-3.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-35.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-39.
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell-lung cancer (KEYNOTE-010): a randomized controlled trial. Lancet. 2016;387(10027):1540-50.
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-33.
Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol. 2013;8(6):803-5.
Senoo S, Ninomiya T, Makimoto G, et al. Rapid and long-term response of pulmonary pleomorphic carcinoma to nivolumab. Intern Med. 2019;58(7):985-9.
Matsumoto Y, Miura T, Horiuchi H, et al. The successful treatment of pulmonary pleomorphic carcinoma with pembrolizumab. Case Rep Oncol. 2017;10(2):752-7.