Corresponding author: Mitsuhiro Matsuo, matsuo@itoigawa-hp.jp
DOI: 10.31662/jmaj.2019-0076
Received: December 23, 2019
Accepted: April 1, 2020
Advance Publication: June 19, 2020
Published: July 15, 2020
Cite this article as:
Matsuo M. Impact of Age-related Diseases on Pulmonary Function Tests in Older Japanese Adults: A Cross-sectional Pilot Study. JMA J. 2020;3(3):251-257.
Introduction: A widely used reference range for pulmonary function testing was derived from middle-aged, healthy, non-smoking adults in Japan. This study examined the effect of age-related diseases on pulmonary function tests for older Japanese adults.
Methods: All patients aged ≥65 years who underwent spirometry before general and orthopedic surgeries in Itoigawa General Hospital (Niigata, Japan) from January 2014 to June 2019 were identified, and their charts were reviewed.
Results: This study included 1050 Japanese patients (median age: 75 years). The median spirometric values of vital capacity, forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and FEV1/FVC in all patients were 2.66 L [interquartile range; 2.24, 3.25], 2.57 L [2.13, 3.13], 1.98 L [1.66, 2.37], and 77.5% [72.2, 81.9], respectively. Multiple regression analyses revealed that spirometric values were significantly affected by age, body height, sex, smoking status, social dependency, dyslipidemia, diabetes, history of heart failure, peripheral artery disease, end-stage renal disease, neuromuscular disease, and psychiatric disorders. Male sex and height were positively correlated with FVC and FEV1. Other factors, such as a history of heart failure, neuromuscular disease, and independent physical activity, were negatively correlated with FVC and FEV1 to almost the same extent as that of age.
Conclusions: These data will provide clinically useful information to accurately interpret pulmonary function test results in older Japanese adults.
Key words: aging, frailty, reference value, spirometry
Pulmonary function testing is considered as the basis for diagnosis in many categories of pulmonary disease (1). Aging causes a loss of functional reserves in many organ systems (2), and respiratory function declines in an age-dependent manner (3). Although there is an increasing number of older patients with respiratory diseases in Japan (4), (5), a widely used reference range for spirometry is mainly derived from middle-aged adults who are healthy non-smokers (3). Older patients often have many types of pre-existing comorbidities and geriatric syndrome. Diversity among age-related diseases should be considered in order to have sufficient understanding of the respiratory functions of older patients.
Pre-anesthetic and pre-surgical assessments include a complete medical history, physiological assessment, current medication, and social status (6). Pulmonary function tests were routinely conducted before general and orthopedic surgeries in the author’s hospital. This study examined the effect of age-related diseases on pulmonary function tests by secondary use of preoperative data evaluated before non-thoracic surgery for patients aged 65 years and older.
This cross-sectional study was approved by the ethics committee of Itoigawa General Hospital (#2019-11) and conducted in accordance with the principles of the Declaration of Helsinki. All patients aged ≥65 years who underwent spirometry before general and orthopedic surgeries in Itoigawa General Hospital from January 2014 to June 2019 were identified, and their charts were reviewed. Exclusion criteria included thoracic surgery; any known respiratory disease; chest X-ray abnormalities, such as infiltrations and pleural effusion; postpneumonectomy; and race other than Japanese. Vital capacity (VC), forced VC (FVC), and forced expiratory volume in 1 second (FEV1) were measured using the standard technique (7) with the HUDAC-77 (Fukuda Denshi Co., Ltd., Tokyo, Japan). Healthcare providers distributed questionnaires on patients’ smoking histories, including information on current smokers, former smokers who had not smoked for more than 1 month, or those who had never smoked. The answer for pack-years was optional. Based on the long-term care insurance (LTCI) law in Japan, the LTCI applicant is assigned to one of the levels of care required (certified support level of 1-2 or care level of 1-5 (2). Patients who were certified at the LTCI support level and who did not require this service were regarded as physically independent. Those who had been diagnosed with dementia were included. Patients who were receiving anti-hypertensive, lipid-lowering, and anti-diabetic drugs were regarded as having hypertension, dyslipidemia, and diabetes mellitus (DM), respectively. Liver cirrhosis with Child–Pugh class B or C was included. End-stage renal disease (ESRD) was defined as an estimated glomerular filtration rate of < 15 mL/min/1.73 m2 or requiring dialysis.
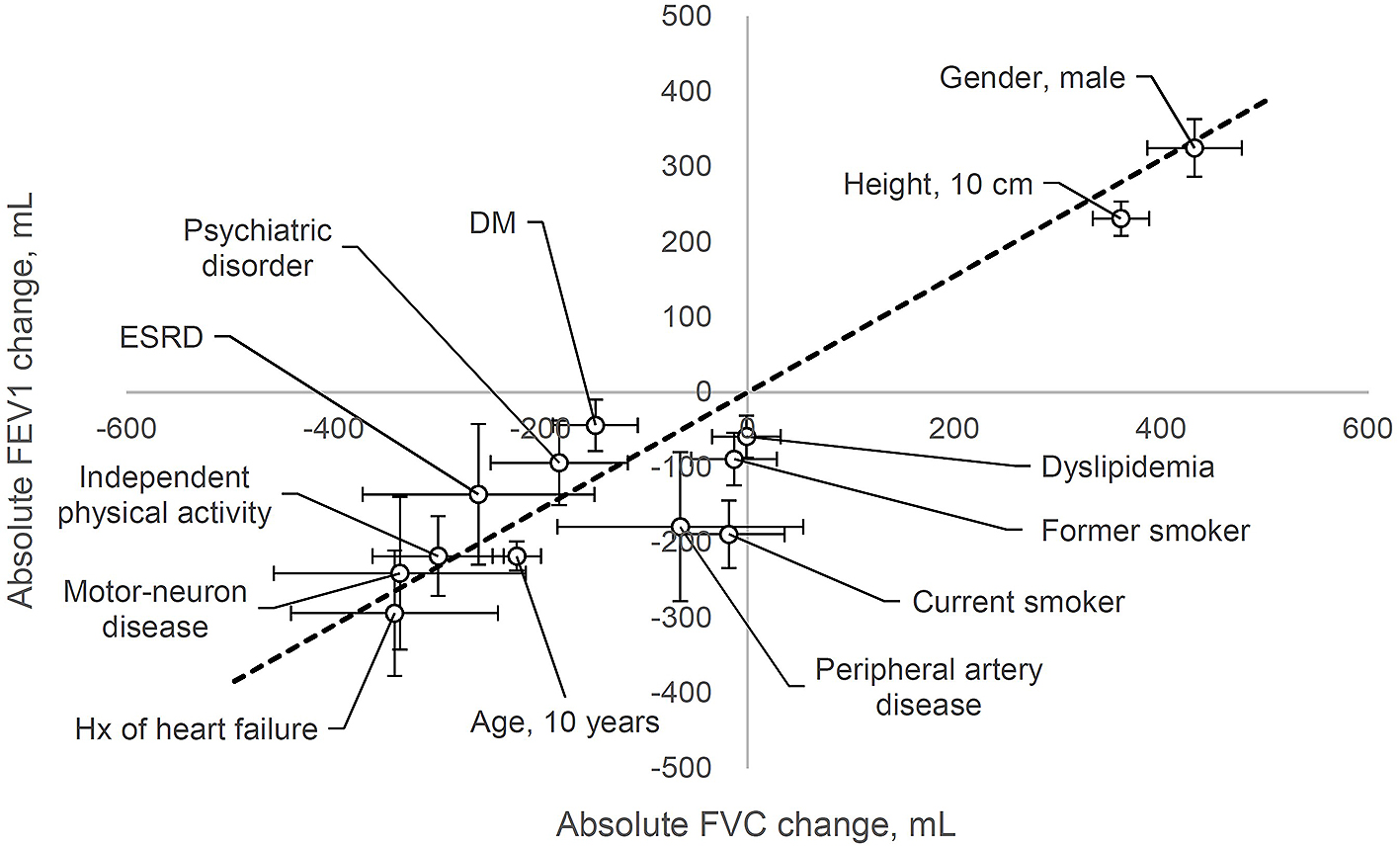
Data are expressed as median [interquartile range]. Multiple regression analysis (simultaneous forced entry method) was conducted to identify the factors affecting spirometric values. The dependent variables were VC, FVC, FEV1, and FEV1/FVC. In this pilot study, the independent variables included all the factors obtained by preoperative evaluation: age, height, weight, sex, smoking status, independent physical activity, hypertension, dyslipidemia, DM, ischemic heart disease, history of heart failure, peripheral artery disease, liver cirrhosis, ESRD, history of stroke, neuromuscular disease, psychiatric disorder, and malignant tumor burden. P < 0.05 was considered statistically significant. Since this was a pilot study for exploratory analysis, no adjustments were made to the test multiplicity. The significant factors obtained above are presented in a scatter plot, which presents the absolute change in FVC on the X axis and the absolute change in FEV1 on the Y axis (Figure 1). All statistical analyses were conducted using EZR, which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (8).
A total of 1210 respiratory function tests were conducted before general and orthopedic surgeries in patients aged ≥65 years in Itoigawa General Hospital over 5.5 years. This study excluded 14 patients due to missing data. Furthermore, 115 patients who had known respiratory diseases, 18 with chest X-ray abnormalities, 12 with pneumonectomy, and 1 who was not Japanese were also excluded. Therefore, the remaining 1050 patients were analyzed, and their characteristics are summarized in Table 1.
Table 1. Patients’ Characteristics in the Survey.
| Male | Female | |
|---|---|---|
| n = 499 | n = 551 | |
| Age, years | 75 [69, 81] | 76 [70, 82] |
| Height, cm | 163 [158, 167] | 150 [145, 154] |
| Weight, kg | 60 [54, 66] | 50 [45, 57] |
| Smoking status | ||
| Never | 191 (38.3) | 514 (93.3) |
| Former | 208 (41.7) | 27 (4.9) |
| Current | 100 (20.0) | 10 (1.8) |
| Independent physical activity | 28 (5.6) | 43 (7.8) |
| Dementia | 9 (1.8) | 27 (4.9) |
| Comorbidity | ||
| Hypertension | 271 (54.3) | 305 (55.4) |
| Dyslipidemia | 111 (22.2) | 193 (35.0) |
| DM | 94 (18.8) | 81 (14.7) |
| Ischemic heart disease | 48 (9.6) | 11 (2.0) |
| History of heart failure | 14 (2.8) | 10 (1.8) |
| PAD | 12 (2.4) | 4 (0.7) |
| Liver cirrhosis | 4 (0.8) | 1 (0.2) |
| ESRD | 7 (1.4) | 11 (2.0) |
| History of stroke | 56 (11.2) | 35 (6.4) |
| Neuromuscular disease | 7 (1.4) | 8 (1.5) |
| Psychiatric disorder | 20 (4.0) | 35 (6.4) |
| Operative indication | ||
| Malignant tumor | 106 (21.2) | 116 (21.1) |
| Respiratory function | ||
| VC, L | 3.25 [2.80, 3.66] | 2.30 [2.00, 2.62] |
| FVC, L | 3.11 [2.69, 3.53] | 2.25 [1.94, 2.53] |
| FEV1, L | 2.33 [1.96, 2.72] | 1.76 [1.51, 2.01] |
| FEV1/FVC, % | 75.7 [70.2, 80.1] | 79.0 [74.6, 83.2] |
| Data are expressed as number (%) or median [interquartile range]. DM, diabetes mellitus; ESRD, end-stage renal disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PAD, peripheral artery disease; VC, vital capacity. | ||
Patients were aged 75 [70, 81] years, and 551 (53%) were women. The number of patients who were current or past smokers was 345 (33%), and the smoking amount was described for 258 patients. The number of pack-years in current smokers was significantly higher than that in former smokers (40 [25, 50] vs 22 [10, 41]). Of the 175 patients with DM, glycemic control was measured in 155, with a median hemoglobin A1c level of 6.6% [6.1, 7.1]. Twenty-four patients had a history of heart failure in whom a chest X-ray revealed cardiomegaly with a cardiopulmonary ratio of 57% [51, 61]. Sixteen patients had neuromuscular diseases, including seven with Parkinson’s or related diseases, two with muscular dystrophy, and two with epilepsy. With regard to psychiatric disorders, 45 patients had a mood disorder, six had schizophrenia, and four had other disorders. The median values of VC, FVC, FEV1, and FEV1/FVC in all patients were 2.66 L [2.24, 3.25], 2.57 L [2.13, 3.13], 1.98 L [1.66, 2.37], and 77.5% [72.2, 81.9], respectively.
Multiple regression analysis was employed to determine the factors affecting spirometric results in older patients. After adjusting for all factors presented in Table 1, spirometric values were significantly affected by age, body height, sex, smoking status, social dependency, dyslipidemia, DM, history of heart failure, peripheral artery disease, ESRD, neuromuscular disease, and psychiatric disorders (Table 2). The factors affecting VC were the same as those affecting FVC. DM, ESRD, and psychiatric disorders were significant only for VC and FVC, but not for FEV1. Contrarily, smoking status and dyslipidemia were significant only for FEV1, but not for VC and FVC.
Table 2. Effect of Age-related Diseases on the Absolute Change in Spirometric Results.
| VC | FVC | FEV1 | FEV1/FVC | |||||
|---|---|---|---|---|---|---|---|---|
| VC, mL | Pvalue | FVC, mL | Pvalue | FEV1, mL | Pvalue | FEV1/FVC, % | Pvalue | |
| Age, 10 years | –210 (–256, –165) | <0.001 | –223 (–269, –177) | <0.001 | –218 (–256, –179) | <0.001 | –1.7 (–2.5, –0.9) | <0.001 |
| Height, 10 cm | 360 (308, 412) | <0.001 | 361 (308, 415) | <0.001 | 231 (186, 275) | <0.001 | –1.7 (–2.6, –0.8) | <0.001 |
| Weight, 10 kg | 17 (–18, 53) | 0.34 | 9 (–27, 45) | 0.63 | 21 (–9, 52) | 0.17 | 0.6 (0, 1.2) | 0.080 |
| Gender, male | 481 (393, 569) | <0.001 | 432 (342, 521) | <0.001 | 325 (251, 400) | <0.001 | –0.6 (–2.2, 0.9) | 0.42 |
| Smoking status | ||||||||
| Former | –14 (–94, 66) | 0.73 | –13 (–95, 68) | 0.75 | –89 (–158, –21) | 0.010 | –2.4 (–3.8, –1.0) | <0.001 |
| Current | –10 (–113, 94) | 0.85 | –18 (–123, 88) | 0.74 | –189 (–278, –101) | <0.001 | –5.6 (–7.4, –3.7) | <0.001 |
| Independent physical activity | –358 (–478, –237) | <0.001 | –299 (–424, –175) | <0.001 | –218 (–322, –113) | <0.001 | 1.5 (–1.0, 3.3) | 0.29 |
| Dementia | 148 (–13, 309) | 0.071 | 71 (–93, 235) | 0.40 | 10 (–127, 148) | 0.88 | –2.1 (–4.9, 0.8) | 0.15 |
| Comorbidity | ||||||||
| Hypertension | –25 (–85, 34) | 0.40 | –39 (–99, 22) | 0.21 | –47 (–97, 4) | 0.071 | –0.7 (–1.8, 0.3) | 0.17 |
| Dyslipidemia | –11 (–75, 53) | 0.74 | –1 (–66, 64) | 0.97 | –59 (–113, 0) | 0.035 | –1.7 (–2.9, –0.6) | 0.002 |
| DM | –159 (–238, –80) | <0.001 | –147 (–228, –67) | 0.003 | –44 (–111, 24) | 0.20 | 2.1 (0.7, 3.4) | 0.004 |
| Ischemic heart disease | –63 (–191, 66) | 0.34 | –65 (–196, 66) | 0.33 | –67 (–177, 42) | 0.23 | –1.1 (–3.4, 1.1) | 0.34 |
| History of heart failure | –331 (–524, –139) | <0.001 | –341 (–537, –145) | <0.001 | –294 (–458, –131) | <0.001 | –1.4 (–4.8, 2.0) | 0.42 |
| PAD | –12 (–241, 217) | 0.92 | –65 (–298, 169) | 0.53 | –179 (–374, 15) | 0.071 | –4.3 (–8.3, –0.3) | 0.034 |
| Liver cirrhosis | –60 (–462, 341) | 0.77 | –122 (–531, 286) | 0.56 | –54 (–395, 287) | 0.75 | 2.6 (–4.4, 9.6) | 0.47 |
| ESRD | –272 (–488, –56) | 0.014 | –260 (–480, –40) | 0.021 | –136 (–320, 47) | 0.15 | 2.6 (–1.1, 6.4) | 0.17 |
| History of stroke | –54 (–154, 46) | 0.29 | –33 (–134, 69) | 0.53 | –24 (–109, 60) | 0.57 | 0.5 (–1.3, 2.2) | 0.58 |
| Neuromuscular disease | –378 (–612, –143) | 0.002 | –336 (–537, –145) | 0.006 | –241 (–441, –42) | 0.018 | 2.4 (–1.7, 6.5) | 0.26 |
| Psychiatric disorder | –280 (–404, –155) | <0.001 | –182 (–312, –52) | 0.006 | –94 (–205, 17) | 0.096 | 0 (–2.3, 2.3) | 0.99 |
| Operative indication | ||||||||
| Malignant tumor | –63 (–130, 5) | 0.069 | –54 (–123, 15) | 0.12 | –46 (–104, 12) | 0.12 | –0.8 (–1.9, 0.4) | 0.21 |
| Multivariate regression analyses were conducted to examine the influence of all factors presented in Table 1 on VC, FVC, FEV1, and FEV1/FVC. Data are expressed as mean (95% confidence interval). DM, diabetes mellitus; ESRD, end-stage renal disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PAD, peripheral artery disease; VC, vital capacity. | ||||||||
Significant parameters from Table 2 are presented in a scatter plot (Figure 1) showing the absolute changes in FVC on the X axis and absolute changes in FEV1 on the Y axis. Figure 1 shows that male sex and height were positively correlated with FVC and FEV1. Other factors, such as a history of heart failure, neuromuscular disease, and independent physical activity, were negatively correlated with FVC and FEV1 to almost the same extent as that of age.
This pilot study addressed the issue of the effect of age-related diseases on pulmonary function tests by secondary use of preoperative data. Multiple regression analysis revealed that in addition to well-accepted factors, such as age, height, sex, and smoking status, many age-related conditions and diseases were additively associated with decreased respiratory function. These data will provide clinically useful information for a precise interpretation of the results of pulmonary function tests in older Japanese patients. Moreover, these profiles will help estimate respiratory function in older patients in whom pulmonary function tests were not conducted before surgery.
A history of heart failure, neuromuscular disease, and independent physical activity affected the spirometric value to almost the same extent as that of age (Figure 1). Although the underlying mechanism involving heart failure is not fully understood, possible explanations include arterial stiffness, severity of mitral valve regurgitation, pulmonary vascular congestion, and enlargement of heart size (9), (10). Impaired muscle strength is one of the most frequent and persistent consequences of aging and neuromuscular disease. Respiratory muscle strength is inversely correlated with pulmonary function, and inspiratory muscle training improves respiratory muscle strength, functional capacity, lung function, and quality of life in many types of diseases (11), (12). In Parkinson’s disease, airway obstruction or restrictive pulmonary dysfunction is highly prevalent, and reduced respiratory muscle strength is observed in patients with early-stage Parkinson’s disease (13), (14). A systematic review revealed the efficacy of levodopa therapy in improving FVC in Parkinson’s disease (15).
Other factors affect respiratory function to a lesser extent than that of the abovementioned factors. Regarding peripheral artery disease, increased brachial-ankle pulse wave velocity (>1400 cm/s) is associated with moderate-to-severe airflow limitation (16). Restrictive lung dysfunction is a common complication in patients with ESRD (17). A decline in VC in patients with mood disorders is reasonable as muscle strength is inversely associated with depressive symptoms (18), (19).
A large-scale epidemiological study revealed that metabolic health is closely associated with impaired lung function (20). In the current study, patients with DM had more reduction in FEV1 than in FVC. This observation is consistent with a recent systematic review, which revealed that patients with DM had a restrictive type of lung pathology (21). Because hemoglobin A1c levels are associated with impairment of restrictive lung function, an evaluation of glycemic control is important to understand spirometric results in patients with DM (22). In the present study, patients with dyslipidemia had a greater reduction in FVC than in FEV1. The underlying mechanism for dyslipidemia inducing a reduction in FVC remains unknown. However, some reports have shown that dyslipidemia (lipoprotein (a) elevation) is associated with a reduction in FVC and FEV1 at almost the same extent (23). Further studies on the types of dyslipidemia and lipid control level are required to elucidate the effect of dyslipidemia.
The main limitation of the present study is its observational, cross-sectional design, which does not permit conclusions concerning causality. Although the exclusion criteria included known respiratory diseases, a substantial number of patients with undiagnosed chronic obstructive pulmonary disease may have been included in this survey (24). Although the amount and duration of smoking contribute to the disease severity (25), pack-years of smoking was not described in many patients. Patients who underwent non-thoracic surgery were selected for the evaluation of pulmonary function in this study, of whom 21.1% of patients had malignancy. Aging-related musculoskeletal abnormalities, such as kyphosis, might greatly affect lung mechanics (26). Although physical independence was based on the Japanese LTCI, frailty (27) and cognitive function (28) were not evaluated in this study. The severity of dementia should be quantified by mental status scales, such as the Mini-Mental State Examination (29). Biomarkers associated with a decline in pulmonary function, such as fibronectin and adiponectin, were not assessed in this study (30). Finally, this study was conducted in a single hospital and included a small number of patients.
In conclusion, this study evaluated the impact of age-related diseases on pulmonary function tests in older Japanese adults. These results will help accurately interpret respiratory function and properly manage geriatric patients under anesthesia; however, further studies are needed to confirm these findings and better understand geriatric syndrome using pulmonary function tests.
None
I thank Ellen Knapp, PhD, and Traci Raley, MS, ELS, from Edanz Group (www.edanzediting.com/ac) for editing the drafts of this manuscript. All analyses were reviewed by Satista Co. Ltd. (https://www.satista.jp/medical/).
MM performed the entire study.
This retrospective cohort study was approved by the ethics committee of Itoigawa General Hospital (#2019-11) and was conducted in accordance with the principles of the Declaration of Helsinki.
As this was a retrospective study, consent for publication was not obtained from the participants.
Ruppel GL, Enright P. Pulmonary function testing. Respir Care. 2012;57(1):165-75.
Matsuo M, Yamagami T, Higuchi A. Impact of age on postoperative complication rates among elderly patients with hip fracture: a retrospective matched study. J Anesth. 2018;32(3):452-6.
Kubota M, Kobayashi H, Quanjer PH, et al. Clinical Pulmonary Functions Committee of the Japanese Respiratory Society. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig. 2014;52(4):242-50.
Iwanaga T, Tohda Y. Epidemiology of asthma in Japan. Nihon Rinsho. 2016;74(10):1603-8. Japanese.
Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the Nippon COPD Epidemiology study. Respirology. 2004;9(4):458-65.
Matsuo M, Tazawa K. Reference range of clinical blood tests in physically independent patients of advanced age with groin hernia in a Japanese hospital. Geriatr Gerontol Int. 2019;19(8):780-5.
Japanese Association of Medical Technologists. Practice of pulmonary function tests. [written in Japanese; author’s translation]. Tokyo: Takayama Co., Ltd.; 2009.
Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-8.
Li L, Hu B, Gong S, et al. Pulmonary function and arterial stiffness in chronic heart failure. Biomed Res Int. 2016;2016:5478394.
Oppenheimer BW, Berger KI, Ali S, et al. Pulmonary vascular congestion: a mechanism for distal lung unit dysfunction in obesity. PLoS One. 2016;11(4):e0152769.
de Medeiros AIC, Fuzari HKB, Rattesa C, et al. Inspiratory muscle training improves respiratory muscle strength, functional capacity and quality of life in patients with chronic kidney disease: a systematic review. J Physiother. 2017;63(2):76-83.
Gomes-Neto M, Saquetto MB, Silva CM, et al. Effects of respiratory muscle training on respiratory function, respiratory muscle strength, and exercise tolerance in patients poststroke: a systematic review with meta-analysis. Arch Phys Med Rehabil. 2016;97(11):1994-2001.
Sabaté M, Rodríguez M, Méndez E, et al. Obstructive and restrictive pulmonary dysfunction increases disability in Parkinson disease. Arch Phys Med Rehabil. 1996;77(1):29-34.
Zhang W, Zhang L, Zhou N, et al. Dysregulation of respiratory center drive (P0.1) and muscle strength in patients with early stage idiopathic Parkinson's disease. Front Neurol. 2019;10:724.
Monteiro L, Souza-Machado A, Valderramas S, et al. The effect of levodopa on pulmonary function in Parkinson's disease: a systematic review and meta-analysi. Clin Ther. 2012;34(5):1049-55.
Oda M, Omori H, Onoue A, et al. Association between airflow limitation severity and arterial stiffness as determined by the brachial-ankle pulse wave velocity: a cross-sectional study. Intern Med. 2015;54(20):2569-75.
Mukai H, Ming P, Lindholm B, et al. Restrictive lung disorder is common in patients with kidney failure and associates with protein-energy wasting, inflammation and cardiovascular disease. PLoS One. 2018;13(4):e0195585.
Wu H, Yu B, Meng G, et al. Both muscle mass and muscle strength are inversely associated with depressive symptoms in an elderly Chinese population. Int J Geriatr Psychiatry. 2017;32(7):769-78.
Park Y, Jung JY, Kim YS, et al. Relationship between depression and lung function in the general population in Korea: a retrospective cross-sectional study. Int J Chron Obstruct Pulmon Dis. 2018;13:2207-13.
Lee HY, Yang HK, Song HJ, et al. Metabolic health is more closely associated with decrease in lung function than obesity. PLoS One. 2019;14(1):e0209575.
Saini M, Kulandaivelan S, Bansal VK, et al. Pulmonary pathology among patients with type 2 diabetes mellitus: an updated systematic review and meta-analysis. Curr Diabetes Rev. 2019.
Sonoda N, Morimoto A, Tatsumi Y, et al. The association between glycemic control and lung function impairment in individuals with diabetes: the Saku study. Diabetol Int. 2018;10(3):213-8.
Lee J, Park HK, Kwon MJ, et al. Decline in lung function is associated with elevated lipoprotein (a) in individuals without clinically apparent disease: A cross-sectional study. Respirology. 2019;24(1):68-75.
Yoshikawa M, Yamamoto Y, Tomoda K, et al. Prevalence of chronic obstructive pulmonary disease in independent community-dwelling older adults: The Fujiwara-kyo study. Geriatr Gerontol Int. 2017;17(12):2421-6.
Løkke A, Lange P, Scharling H, et al. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61(11):935-9.
Lorbergs AL, O'Connor GT, Zhou Y, et al. Severity of kyphosis and decline in lung function: the Framingham study. J Gerontol A Biol Sci Med Sci. 2017;72(5):689-94.
Mills DE, Johnson MA, Barnett YA, et al. The effects of inspiratory muscle training in older adults. Med Sci Sports Exerc. 2015;47(4):691-7.
Melo SMD, Oliveira LA, Wanderley JLF, et al. Evaluating the extremely elderly at a pulmonary function clinic for the diagnosis of respiratory disease: frequency and technical quality of spirometry. J Bras Pneumol. 2019;45(4):e20180232.
Queiroz RS, Faria LMA, Carneiro JAO, et al. Age and mini-mental state examination score can predict poor-quality spirometry in the elderly: a cross-sectional study. Clinics (Sao Paulo). 2018;73:e374.
Shibata Y, Inoue S, Watanabe M. Impact of reduced pulmonary function in the Japanese general population: Lessons from the Yamagata-Takahata study. Respir Investig. 2019;57(3):220-6.