Corresponding author: Makoto Suzuki, msuzuki@hoshinooka-cvc.com
DOI: 10.31662/jmaj.2022-0098
Received: May 3, 2022
Accepted: June 30, 2022
Advance Publication: September 26, 2022
Published: October 17, 2022
Cite this article as:
Suzuki M, Tomoike H, Dai Z, Hosoda T, Sumiyoshi T, Hosoda S, Isobe M. Polyvascular Disease and the Incidence of Cancer in Patients with Coronary Artery Disease. JMA J. 2022;5(4):498-509.
Introduction: Based on the possible relation of atherosclerotic cardiovascular disease to the development of cancer, we examined whether polyvascular disease, as a surrogate marker of the severity of atherosclerosis, is associated with the incidence of cancer in patients with coronary artery disease (CAD).
Methods: A total of 8,856 patients with CAD between January 2009 and July 2014 were eligible for this observational study. Two cohorts were established based on the presence or absence of polyvascular disease (i.e., polyvascular disease and CAD only) and tracked for the incidence of cancer and all causes of death. Polyvascular disease was defined when accompanied by diagnosed aortic and/or peripheral arterial disease or other arterial diseases at enrollment.
Results: With a median follow-up of 1,095 d, the incidence of cancer was markedly higher in the cohort of 716 patients with polyvascular disease than in the cohort of 8,140 patients with CAD only (8.8% vs. 4.9%, P = 0.0001). A large difference in the incidence of cancer was also found in accordance with a number of the coexisting vascular disease with CAD. With the adjustment of shared common risks, polyvascular disease was an independent contributor to the incidence of cancer (hazard ratio, 1.362; 95% confidence interval [CI], 1.029-1.774). In a total of 548 patients (6.2% of participants) died during follow-up, and all-cause, cardiovascular, and cancer mortalities were all higher in the cohort with polyvascular disease than in the cohort with CAD only.
Conclusion: The presence of polyvascular disease may be associated with the incidence of cancer in patients with CAD, implying a pivotal role of the severity of atherosclerosis in cancer development (ClinicalTrials.gov. number: NCT04198896).
Key words: atherosclerosis, cancer, cardio-oncology, polyvascular disease, coronary artery disease, cohort study
Healthcare epidemiology has been contemporarily validated to acquire sustainable health longevity in the prevention and treatment of atherosclerotic cardiovascular disease and cancer, which are the two leading causes of death among non-communicable diseases (1). Unhealthy lifestyle behaviors and their associated diseases, including obesity, tobacco smoking, hypertension, and diabetes mellitus, among others, may share many common risk factors for the development of both atherosclerotic cardiovascular disease and cancer, with the possibility of overlapping causes for both diseases (2), (3). Recent international perspectives further endorse the practical impact of various cardiovascular diseases on cancer development (4), (5). We also reported the possibility of an association between atherosclerotic cardiovascular disease itself and the incidence of cancer (6). Based on these findings, we then investigated how the severity of atherosclerosis may affect cancer development. The presence of polyvascular disease, namely, the coexistence of atherosclerotic diseases in two or more vascular trees, is now well-recognized as a high-risk phenotype of atherosclerosis for subsequent cardiovascular events (7), (8). Thus, the present study evaluated whether the presence of polyvascular disease, as a surrogate marker of the severity of atherosclerosis, is associated with the incidence of cancer in patients with coronary artery disease (CAD) using the Sakakibara Health Integrative Profile (SHIP) surveillance system.
As previously reported (6), the SHIP surveillance system was launched in 2006 to annually track subsequent clinical incidents requiring hospitalization, including not only cardiovascular diseases but also non-cardiovascular diseases such as cancer, pneumonia, and fracture, with the purpose of improving healthy life expectancy in patients referred to the Sakakibara Heart Institute. Since 2009, medical records of invasive cardiovascular treatments such as catheter and/or surgical interventions in our institute were also tracked. In the present study, a total of 9,092 patients with CAD who were identified from the SHIP surveillance system between January 2009 and July 2014 were extracted and followed-up by the end of October, 2019.
As previously reported (6), CAD includes acute coronary syndrome, previous myocardial infarction, and stable ischemic heart disease. Acute coronary syndrome was diagnosed according to the urgent algorithm composed of symptomatic abnormal ST segments in the 12-lead electrocardiogram, with abnormal cardiac enzyme spillover, and an urgent coronary angiogram. A previous myocardial infarction and stable ischemic heart disease were diagnosed by invasive and non-invasive cardiac examinations for the assessment of myocardial ischemia and viability. The appropriate use of invasive coronary therapies (i.e., percutaneous coronary intervention and/or coronary bypass surgery) was decided by the heart team. In accordance with the previous studies (7), (8), polyvascular disease was defined as the presence of any diagnosed arterial diseases, including aortic disease, peripheral arterial disease, and other arterial diseases. A diagnosis of aortic disease, which included acute aortic syndrome and/or aortic aneurysm, was made by imaging modalities, mainly, computed tomography, or medical records, including invasive treatments such as arterial bypass graft and/or endovascular replacement. Peripheral arterial disease was diagnosed with the following findings: symptomatic lower limb ischemia, such as intermittent claudication with an ankle-brachial index less than 0.9 and with an abnormal vascular echocardiogram or medical records of any vascular treatment, including amputation. Other arterial diseases were any atherosclerotic cardiovascular disease, except aortic and/or peripheral arterial disease, including atherosclerotic renal artery stenosis, subclavian artery stenosis, and carotid artery disease, mainly diagnosed by measurements with duplex ultrasonography and/or computed tomography angiography according to the established guideline (9). Briefly, atherosclerotic renal artery stenosis was defined with a duplex Doppler peak systolic velocity above 200 cm/s or more than 70% stenosis at the renal ostial arteries, subclavian artery stenosis by a duplex of high systolic velocity, occasionally with a reversal flow in an ipsilateral vertebral artery, followed and an angiographic significant subclavian stenosis, and carotid artery stenosis by more than 50% stenosis based on the North American Symptomatic Carotid Endarterectomy Trial definition. If aortic and/or peripheral arterial disease were present with any other arterial diseases, they were diagnosed as aortic and/or peripheral arterial disease. Based on the assumption that the presence of polyvascular disease is a surrogate marker of the severity of atherosclerosis, the study patients were categorized into the two cohorts with and without polyvascular disease, namely, the cohort with polyvascular disease and with CAD only, at enrollment in the SHIP surveillance system to evaluate the association between the severity of atherosclerosis and cancer development. Furthermore, after dividing the cohort with polyvascular disease into the two subsets in accordance with the coexistence of one vascular disease (i.e., aortic disease, peripheral arterial disease, or other arterial diseases) or two vascular diseases (i.e., both aortic and peripheral arterial diseases), the incidence of cancer was also evaluated. The present study did not include cerebrovascular disease in the category of polyvascular disease because our institute did not treat cerebrovascular disease patients and, thereby, had practical difficulties classifying causes of cerebrovascular disease into ischemic (i.e., lacunar infarction, atheroma-embolic infarction, and cardio-embolic infarction) or hemorrhagic stroke. Demographic and clinical characteristics, including the presence of hypertension, dyslipidemia, diabetes mellitus, chronic kidney disease, and chronic obstructive pulmonary diseases, in accordance with the universal definitions (10), (11), were examined, and a history of cigarette smoking was also confirmed. Using the SHIP surveillance system, medical records of invasive therapies by the end of follow-up and medications at the final follow-up were extracted. All organ sites of cancer and causes of death during the follow-up periods were also identified. As one of the regulations for the managements of the SHIP surveillance system, wherein there is a lack of any contact to the patients as to their clinical status for two years, the tracking was automatically discontinued.
Continuous variables are presented as means ± standard deviation or medians with interquartile range. Differences between the two cohorts with CAD only and polyvascular disease, or the two subsets in the cohort with polyvascular disease were compared using Student’s t-test or the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Differences in three or more corresponding variables were assessed by one-way analysis of variance for continuous variables and Pearson’s test for categorical variables. The cumulative probability of cancer development or all-cause death during follow-up was examined using Kaplan-Meier curves with the log-rank test and/or the Wilcoxon test for comparison between the two cohorts with CAD only and polyvascular disease. The incidence of cancer in the subsets of the cohort with polyvascular disease were also compared with the cohorts with CAD only using Kaplan-Meier curves with the log-rank test and/or the Wilcoxon test. To assess the association of polyvascular disease with the incidence of cancer, adjustment for differences in clinical characteristics was performed using propensity score matching, with measurement of the C-statistic to evaluate the ability to control for confounding bias with a range of 0 to 1, with a higher value indicating well-controlled. A Cox proportional hazards regression analysis was carried out to assess the association of multiple risk co-factors with the incidence of cancer and also to test for the interactions of different clinical characteristics between the two cohorts with CAD only and polyvascular disease for the presence of polyvascular disease on the incidence of cancer. A two-tailed P-value of <0.05 was considered to indicate a significant difference. Statistical analyses were performed using JMP version 11.2.1. (SAS Institute Japan Inc. Tokyo, Japan). The SHIP surveillance system complied with the Declaration of Helsinki and was approved by the local ethics committee on September 16th, 2003, in the Sakakibara Heart Institute (no. 11000304) (6). All patients gave written, informed consent to be included in this surveillance system.
Of the 9,092 patients (74% male, age 70 ± 12 years) with CAD, a total of 8,856 patients were eligible for the present study because 106 patients had cancers on enrollment in the SHIP surveillance system, and 130 patients have not had any contact to us after enrollment. A median follow-up was 1,095 d with a maximum of 3,527 d. These patients were categorized into the two cohorts, 8,140 with CAD only and 716 with polyvascular disease, to compare the incidence of cancer (Figure 1). Table 1 shows the demographic and clinical characteristics of the patients at enrollment, records of invasive coronary therapies by the end of follow-up, and final medications on both crude and propensity score-matched analyses. In the crude analysis, demographic characteristics showed more elderly patients, more male patients, and high prevalence of hypertension, chronic kidney disease, chronic obstructive pulmonary disease, previous stroke, and cigarette smokers in the cohort with polyvascular disease than that in the cohort with CAD only. As to the medical history, previous myocardial infarction and heart failure were observed more frequently in the cohort with polyvascular disease than that in the cohort with CAD only. During the follow-up periods, 4,514 patients, 51% of the study patients, underwent percutaneous coronary intervention and/or coronary artery bypass grafting for the treatment of CAD, and their proportions were not different between the two cohorts with CAD only and polyvascular disease (51% vs. 54%, P = 0.0723), except for a higher percentage of coronary bypass grafting in the cohort with polyvascular disease than that in the cohort with CAD only. Of those treated with invasive coronary therapies, 308 of 4,126 patients in the cohort with CAD only and 32 of 388 patients in the cohort with polyvascular disease underwent both percutaneous coronary intervention and coronary artery bypass grafting (7% vs. 8%, P = 0.5469). Aortic disease was the main coexisting vascular disease in the cohort with polyvascular disease, and peripheral arterial disease was next. As for the final medications, differences in prescriptions of anticoagulants, diuretics, and statins were observed between the two cohorts (Table 1).
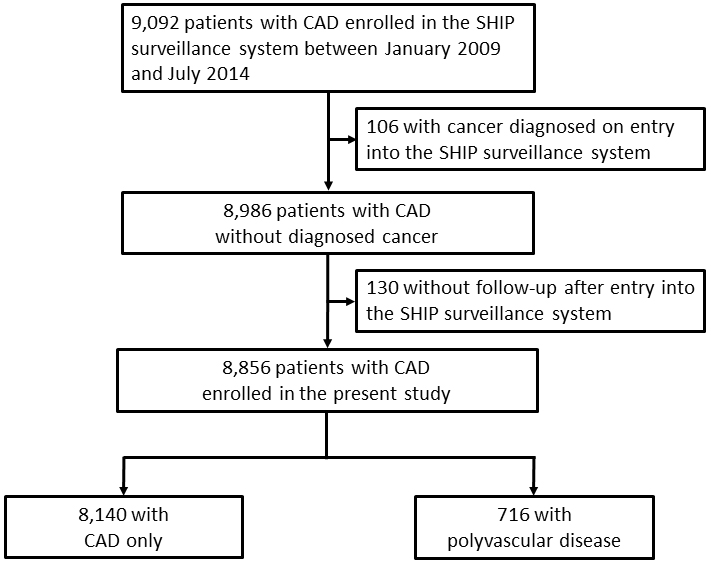
Table 1. Clinical Characteristics of the Two Cohorts, with Coronary Artery Disease (CAD) Only and with a Polyvascular Disease.
| Characteristic | Overall | Crude analysis | P-value | Propensity score-matched analysis | P-value | ||
|---|---|---|---|---|---|---|---|
| CAD only | Polyvascular disease | CAD only | Polyvascular disease | ||||
| N = 8856 | N = 8140 | N = 716 | N = 713 | N = 713 | |||
| Age, y | 70 ± 11 (71, 63-78) |
69 ± 12 (71, 62-77) |
74 ± 9 (75, 69-80) |
<0.0001 | 74 ± 9 (75, 69-80) |
74 ± 9 (75, 69-80) |
0.9416 |
| Male, no. (%) | 6659 (74.1) |
5980 (73.5) |
579 (80.9) |
<0.0001 | 577 (80.9) |
576 (80.8) |
0.9463 |
| Body mass index, kg/m2 | 24 ± 3 (24, 22-26) |
24 ± 3 (24, 22-26) |
24 ± 3 (24, 22-26) |
0.5601 | 24 ± 9 (24, 22-26) |
25 ± 8 (24, 22-26) |
0.8306 |
| Hypertension, no. (%) | 4915 (55.5) |
4444 (54.6) |
471 (65.8) |
<0.0001 | 475 (66.6) |
468 (65.6) |
0.6953 |
| Dyslipidemia, no. (%) | 5021 (56.7) |
4637 (57.0) |
384 (53.6) |
0.0907 | 434 (60.9) |
383 (53.7) |
0.0063 |
| Diabetes mellitus, no. (%) | 2384 (26.9) |
2174 (26.7) |
210 (29.3) |
0.1351 | 220 (30.9) |
209 (29.3) |
0.5253 |
| Chronic kidney disease, no. (%) | 949 (10.7) |
785 (9.6) |
164 (22.9) |
<0.0001 | 154 (21.6) |
161 (22.6) |
0.7018 |
| Chronic obstructive pulmonary disease, no. (%) | 159 (1.8) |
127 (1.6) |
32 (4.5) |
<0.0001 | 29 (4.1) |
29 (4.1) |
1 |
| Previous stroke, no. (%) | 225 (2.5) |
188 (2.3) |
37 (5.2) |
<0.0001 | 22 (3.1) |
36 (5.1) |
0.0805 |
| Cigarette smoking, no. (%) | 4300 (48.9) |
3840 (47.2) |
460 (64.3) |
<0.0001 | 460 (64.5) |
457 (64.1) |
0.8683 |
| Stable ischemic heart disease, no. (%) | 8404 (94.9) |
7714 (94.8) |
690 (96.4) |
0.0628 | 675 (94.7) |
687 (96.3) |
0.1590 |
| Acute coronary syndrome, no. (%) | 452 (5.1) |
426 (5.2) |
26 (3.6) |
0.0628 | 38 (5.3) |
26 (3.7) |
0.1248 |
| Previous myocardial infarction, no. (%) | 2693 (30.4) |
2429 (29.8) |
264 (36.9) |
0.0001 | 255 (35.8) |
263 (36.9) |
0.6569 |
| History of heart failure, no. (%) | 388 (4.4) |
345 (4.2) |
43 (6.0) |
0.0354 | 30 (4.2) |
42 (5.9) |
0.1830 |
| Left ventricular ejection fraction, % | 57 ± 10 (61, 54-64) |
58 ± 10 (61, 54-64) |
57 ± 11 (61, 53-64) |
0.3917 | 57 ± 11 (61, 53-64) |
57 ± 11 (61, 53-64) |
0.7928 |
| Percutaneous coronary intervention, no. (%) | 3682 (41.6) |
3412 (41.9) |
270 (37.7) |
0.0296 | 339 (47.6) |
267 (37.5) |
0.0001 |
| Coronary artery bypass grafting, no. (%) | 1172 (13.2) |
1022 (12.6) |
150 (21.0) |
<0.0001 | 98 (13.7) |
150 (21.0) |
0.0004 |
| History of aortic dissection, no. (%) | 52 (0.6) |
- | 52 (7.3) |
- | - | 52 (7.3) |
- |
| Aortic aneurysm, no. (%) | 343 (3.9) |
- | 343 (47.9) |
- | - | 342 (48.0) |
- |
| Peripheral arterial disease, no. (%) | 273 (3.1) |
- | 273 (38.1) |
- | - | 271 (38.0) |
- |
| Other arterial diseases, no. (%) | 70 (0.8) |
- | 70 (9.8) |
- | - | 70 (9.8) |
- |
| Medications at final follow-up | |||||||
| Antiplatelets, no. (%) | 4707 (53.2) |
4322 (53.1) |
385 (53.8) |
0.7285 | 386 (54.1) |
383 (53.7) |
0.9154 |
| Anticoagulants, no. (%) | 1449 (16.3) |
1302 (16.0) |
147 (20.5) |
0.0017 | 135 (18.9) |
147 (20.6) |
0.4646 |
| Renin-angiotensin inhibitors, no. (%) | 3342 (37.7) |
3049 (37.5) |
293 (40.9) |
0.0704 | 294 (41.2) |
291 (40.8) |
0.9143 |
| Beta-blockers, no. (%) | 3826 (43.2) |
3497 (43.0) |
329 (46.0) |
0.1250 | 331 (46.4) |
329 (46.1) |
0.9576 |
| Calcium-channel blockers, no. (%) | 3448 (38.9) |
3155 (38.8) |
293 (40.9) |
0.2631 | 273 (38.3) |
291 (40.8) |
0.3572 |
| Diuretics, no. (%) | 1030 (11.6) |
908 (11.2) |
122 (17.0) |
<0.0001 | 106 (14.9) |
122 (17.1) |
0.2784 |
| Statins, no. (%) | 4239 (47.9) |
3923 (48.2) |
316 (44.1) |
0.0386 | 332 (46.6) |
315 (44.2) |
0.3947 |
| Length of follow-up, days | 1072 ± 503 (1095, 719-1469) |
1074 ± 505 (1094, 720-1470) |
1050 ± 484 (1098, 700-1467) |
0.2317 | 1072 ± 493 (1099, 701-1469) |
1049 ± 484 (1096, 697-1467) |
0.3615 |
| Plus-minus values are means ± standard deviation, with the median and interquartile range. CAD, coronary artery disease. Body mass index is the weight divided by the square of the height. | |||||||
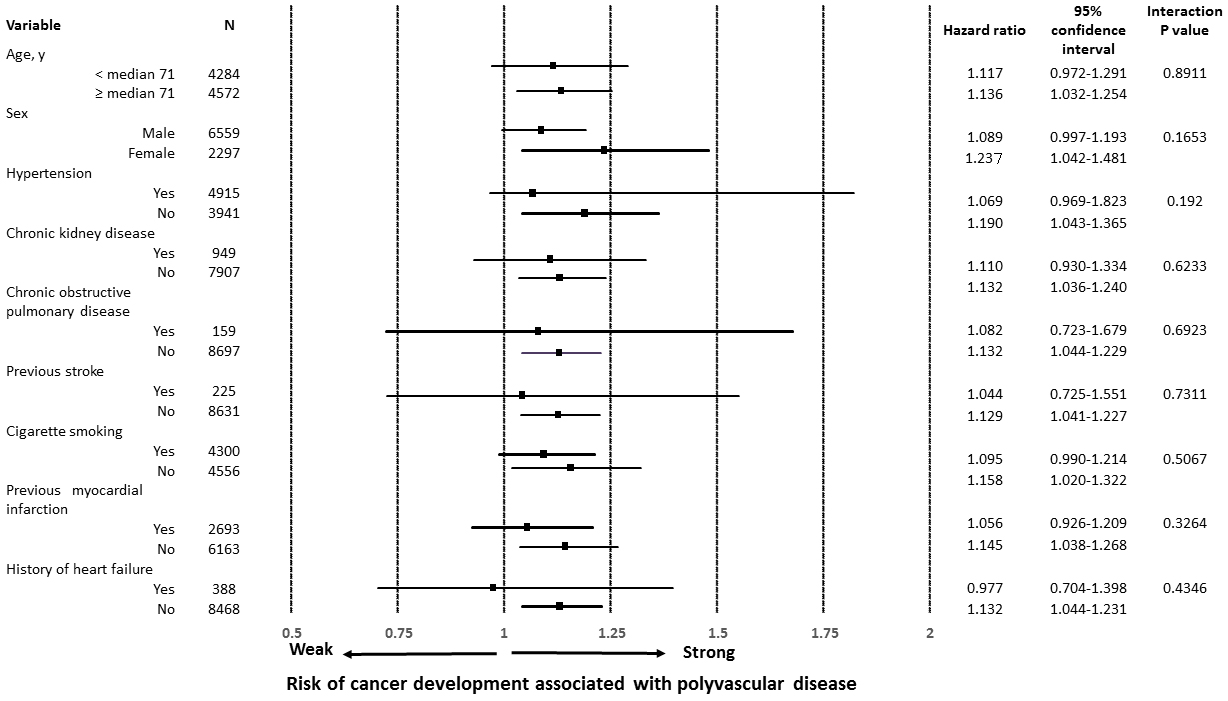
A number of patients with newly diagnosed cancer was 396 in the cohort with CAD only and 63 in the cohort with polyvascular disease (4.9% vs. 8.8%, P < 0.0001), indicating a difference in the cumulative incidence of cancer between the two cohorts (Figure 2A). After a well-balanced propensity score matching procedure for confounding factors among the baseline characteristics, including age, male sex, hypertension, chronic kidney disease, chronic obstructive pulmonary disease, and cigarette smoking, previous myocardial infarction, and heart failure, with a C-statistic of 0.7 (Table 1), a higher incidence of cancer was still found in the cohort with polyvascular disease (Figure 2B). Even when limited to patients treated with invasive coronary therapies, a difference in the incidence of cancer was relatively unchanged between the two cohorts (4.7% vs. 8.3%, P = 0.0022). The distribution of organ sites of cancer development was not significantly different between the two cohorts (Table 2).On both univariate and multivariate Cox proportional hazards regression analyses adjusting for age, male sex, hypertension, chronic kidney disease, chronic obstructive pulmonary disease, and cigarette smoking, the presence of polyvascular disease was one of the principal contributors to the incidence of cancer, with a 36% higher relative risk than that of the cohort with CAD only (Table 3). Interactions of different clinical characteristics between the two cohorts were not significant for the presence of polyvascular disease on the incidence of cancer (Figure 3). A total of 548 patients (6.2% of the study patients) died during follow-up, 197 (2.2%) died due to cardiovascular disease, and 123 died (1.4%) due to cancer. All-cause mortality (15.2% vs. 5.4%, P < 0.0001), cardiovascular mortality (5.2% vs. 2%, P < 0.0001), and cancer mortality (2.5% vs. 1.3%, P = 0.0073) were all higher in the cohort with polyvascular disease than that in the cohort with CAD only, showing a large difference with time (Supplementary Figure S1).
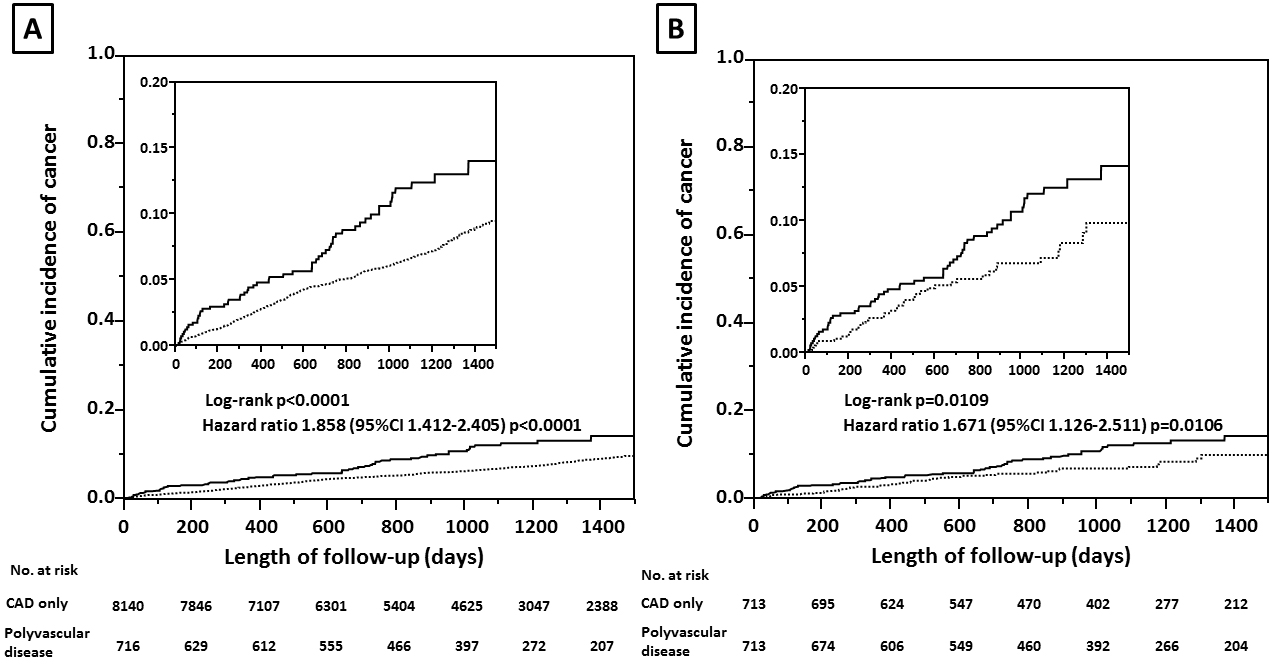
Table 2. Organ Sites of Cancer in the Two Cohorts: with a Coronary Artery Disease (CAD) Only and with a Polyvascular Disease.
| Cancer site, no. (%) | CAD only N = 396 |
Polyvascular disease N = 63 |
P-value |
|---|---|---|---|
| Oral cavity and pharynx | 12 (3.0) | 2 (3.2) | 0.9507 |
| Digestive system | 183 (46.2) | 28 (44.4) | 0.7937 |
| Esophagus | 12 (3.0) | 3 (4.8) | 0.4727 |
| Stomach | 64 (16.2) | 6 (9.5) | 0.1734 |
| Small intestine | 4 (1.0) | 0 (0) | 0.4230 |
| Colon | 52 (13.1) | 7 (11.1) | 0.6563 |
| Rectum | 6 (1.5) | 1 (1.6) | 0.9654 |
| Anus, anal canal & anorectum | 0 (0) | 0 (0) | 1.0000 |
| Liver and intrahepatic bile duct | 22 (5.6) | 8 (12.7) | 0.0331 |
| Gallbladder and biliary tract | 10 (2.5) | 1 (1.6) | 0.6512 |
| Pancreas | 12 (3.0) | 2 (3.2) | 0.9507 |
| Other digestive system | 1 (0.3) | 0 (0) | 0.6897 |
| Respiratory system | 57 (14.4) | 11 (17.5) | 0.5245 |
| Larynx | 3 (0.8) | 0 (0) | 0.4882 |
| Lung and bronchus | 53 (13.4) | 11 (17.5) | 0.3856 |
| Other respiratory organs | 1 (0.3) | 0 (0) | 0.6897 |
| Bones and joints | 2 (0.5) | 0 (0) | 0.5719 |
| Soft tissues (including heart) | 0 (0) | 0 (0) | 1.0000 |
| Skin | 9 (2.3) | 1 (1.6) | 0.7292 |
| Breast | 12 (3.0) | 0 (0) | 0.1615 |
| Genital system | 64 (16.2) | 9 (14.3) | 0.7053 |
| Uterine cervix and corpus | 5 (1.3) | 0 (0) | 0.3698 |
| Ovary | 1 (0.3) | 0 (0) | 0.6897 |
| Prostate | 56 (14.1) | 9 (14.3) | 0.9757 |
| Other genital organs | 2 (0.5) | 0 (0) | 0.5719 |
| Urinary system | 37 (9.3) | 7 (11.1) | 0.6580 |
| Urinary bladder | 21 (5.3) | 4 (6.3) | 0.7340 |
| Kidney and renal pelvis | 14 (3.5) | 2 (3.2) | 0.8847 |
| Ureter and other urinary organs | 2 (0.5) | 1 (1.6) | 0.3221 |
| Eye and orbit | 0 (0) | 0 (0) | 1.0000 |
| Brain and nervous system | 1 (0.3) | 1 (1.6) | 0.1352 |
| Endocrine system | 0 (0) | 0 (0) | 1.0000 |
| Lymphoma | 9 (2.3) | 2 (3.2) | 0.6637 |
| Myeloma | 4 (1.0) | 1 (1.6) | 0.6818 |
| Leukemia | 6 (1.5) | 0 (0) | 0.3254 |
| Other and unspecified primary sites | 0 (0) | 1 (1.6) | 0.0121 |
| Values are numbers (% of each cohort). CAD, coronary artery disease. Pearson, P = 0.3807 between the two cohorts. |
|||
Table 3. Cox-proportional Hazard Analysis for the Incidence of Cancer.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Chi-squared | Hazard ratio (95% confidence interval) |
P-value | Chi-squared | Hazard ratio (95% confidence interval) |
P-value | |
| Age, for each 1 year | 122.355 | 1.055 (1.044-1.066) |
<0.0001 | 120.687 | 1.057 (1.046-1.068) |
<0.0001 |
| Male | 25.016 | 1.813 (1.423-2.343) |
<0.0001 | 34.475 | 2.113 (1.630-2.770) |
<0.0001 |
| Hypertension | 8.221 | 1.316 (1.090-1.593) |
0.0041 | 2.301 | 1.159 (0.958-1.407) |
0.1293 |
| Chronic kidney disease | 19.491 | 1.785 (1.393-2.258) |
<0.0001 | 4.403 | 1.312 (1.019-1.670) |
0.0359 |
| Chronic obstructive pulmonary disease | 3.969 | 1.806 (1.011-2.953) |
0.0464 | 0.710 | 1.270 (0.708-2.085) |
0.3995 |
| Cigarette smoking | 3.325 | 1.186 (0.987-1.425) |
0.0682 | 0.179 | 1.043 (0.858-1.270) |
0.0672 |
| Polyvascular disease | 17.952 | 1.858 (1.412-2.405) |
<0.0001 | 4.639 | 1.362 (1.029-1.774) |
0.0312 |
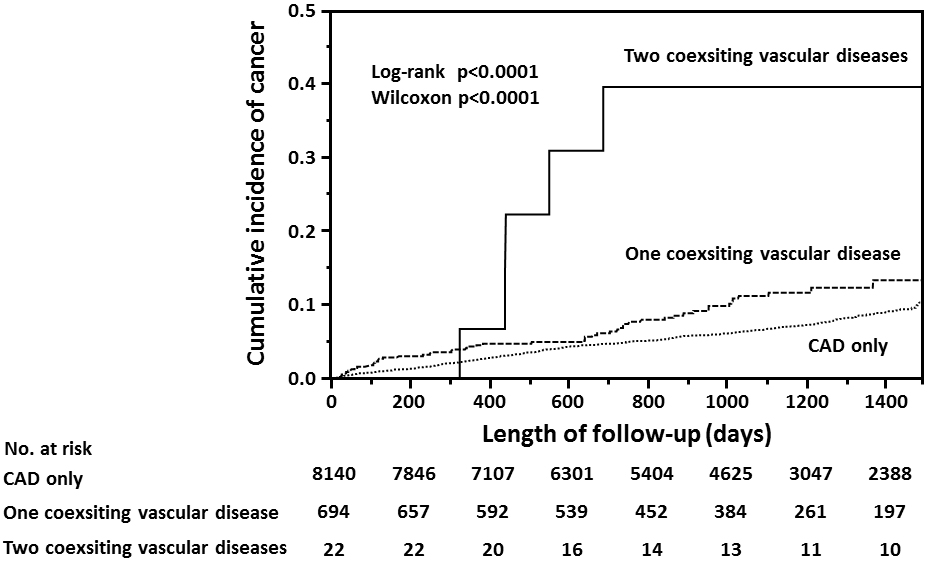
In the cohort with polyvascular disease, half of the patients had aortic disease, one-third had peripheral arterial disease, and 22 had both aortic and peripheral arterial diseases (Supplementary Table S1). By the end of follow-up, cancer was found in 28 of 373 patients (7.5%) with aortic disease, 27 of 251 (10.8%) with peripheral arterial disease, six of 22 (27.3%) with both aortic and peripheral arterial diseases, and two of 70 (2.9%) with other arterial diseases. Between the cohorts with CAD only and the two subsets of polyvascular disease (i.e., one or two coexisting vascular diseases) (Table 4), a large difference in the incidence of cancer was found in accordance with a number of the coexisting vascular disease (Figure 4).
Table 4. Clinical Characteristics of the Subset Cohorts of Polyvascular Disease.
| Characteristic | one coexisting vascular disease N = 694 |
two coexisting vascular diseases N = 22 |
P value |
|---|---|---|---|
| Age, y | 74 ± 9 (75, 69-80) |
76 ± 8 (74, 72-83) |
0.1739 |
| Male, no. (%) | 562 (81.0) | 17 (77.3) | 0.5906 |
| Body mass index, kg/m2 | 24 ± 3 (24, 22-26) |
25 ± 4 (24, 22-26) |
0.1761 |
| Hypertension, no. (%) | 460 (66.3) | 11 (50.0) | 0.1688 |
| Dyslipidemia, no. (%) | 373 (53.8) | 11 (50.0) | 0.8291 |
| Diabetes mellitus, no. (%) | 206 (29.7) | 4 (18.2) | 0.3421 |
| Chronic kidney disease, no. (%) | 159 (22.9) | 5 (22.7) | 1.0000 |
| Chronic obstructive pulmonary disease, no. (%) | 30 (4.3) | 2 (9.1) | 0.2575 |
| Previous stroke, no. (%) | 36 (5.2) | 1 (4.6) | 1.0000 |
| Cigarette smoking, no. (%) | 446 (64.3) | 14 (63.6) | 1.0000 |
| Stable ischemic heart disease, no. (%) | 668 (96.3) | 22 (100) | 1.0000 |
| Acute coronary syndrome, no. (%) | 26 (3.7) | 0 (0) | 1.0000 |
| Previous myocardial infarction, no. (%) | 251 (36.2) | 13 (59.1) | 0.0411 |
| History of heart failure, no. (%) | 41 (5.9) | 2 (9.1) | 0.3856 |
| Left ventricular ejection fraction, % | 57 ± 11 (61, 53-64) |
50 ± 14 (52, 44-62) |
0.0059 |
| Percutaneous coronary intervention, no. (%) | 257 (37.0) | 13 (59.1) | 0.0441 |
| Coronary artery bypass grafting, no. (%) | 147 (21.2) | 3 (13.6) | 0.5942 |
| History of aortic dissection, no. (%) | 51 (7.4) | 1 (4.6) | 1.0000 |
| Aortic aneurysm, no. (%) | 322 (46.4) | 21 (95.4) | <0.0001 |
| Peripheral artery disease, no. (%) | 251 (36.2) | 22 (100) | <0.0001 |
| Other arterial diseases, no. (%) | 70 (10.1) | - | - |
| Medications at final follow-up Antiplatelets, no. (%) | 372 (53.6) | 13 (59.1) | 0.6688 |
| Anticoagulants, no. (%) | 142 (20.5) | 5 (22.7) | 0.7896 |
| Renin-angiotensin inhibitors, no. (%) | 284 (40.9) | 9 (40.9) | 1.0000 |
| Beta-blockers, no. (%) | 322 (46.4) | 7 (31.8) | 0.1983 |
| Calcium-channel blockers, no. (%) | 285 (41.1) | 8 (36.4) | 0.8246 |
| Diuretics, no. (%) | 116 (16.7) | 6 (27.3) | 0.2427 |
| Statins, no. (%) | 308 (44.4) | 8 (36.4) | 0.5184 |
| All-cause death, no. (%) | 103 (14.8) | 6 (27.3) | 0.1272 |
| Cardiovascular death, no. (%) | 36 (5.2) | 1 (4.6) | 1.0000 |
| Cancer death, no. (%) | 16 (2.3) | 2 (9.1) | 0.1025 |
| Length of follow-up, days | 1046 ± 482 (1096, 697-1464) |
1174 ± 549 (1199, 666-1600) |
0.2223 |
| Plus-minus values are means ± standard deviation, with the median and interquartile range. CAD, coronary artery disease. Body mass index is the weight divided by the square of the height. | |||
The results showed the significantly high incidence of cancer in patients with CAD, along with the presence of polyvascular disease, as compared to that in patients with CAD only. A large difference in the incidence of cancer was also found in accordance with a number of the coexisting vascular disease with CAD. An adjustment of shared common risks between atherosclerosis and cancer demonstrated polyvascular disease as an independent contributor to the incidence of cancer. These findings imply that the presence of polyvascular disease, as a surrogate marker of the severity of atherosclerosis, would likely concern cancer development in patients with CAD.
Based on our previous study that showed a higher incidence of cancer in patients with atherosclerotic cardiovascular disease than that in those with non-atherosclerotic cardiovascular disease (6), the present study addressed the next question, whether the severity of atherosclerosis may further impact cancer development. It was assumed that the presence of polyvascular disease is an indicator of the severity of atherosclerosis in patients with CAD. Because of the heterogeneous features of atherosclerosis, which are mediated through various mechanisms, including endothelial dysfunctions, oxidized lipoproteins, inflammations, and genetic associations, et al., based on long-term unhealthy lifestyle behaviors (12), precise quantification of the severity of atherosclerosis has been limited clinically. So far, cumulative verifications of increased adverse cardiovascular events in the setting of polyvascular disease confirm that this phenotype represents the severity of atherosclerosis (8), (13). In fact, cardiovascular mortality was higher in the cohort with polyvascular disease than that in the cohort with CAD only in the present study. As one of the considerations, even though comorbidities were carefully evaluated using the SHIP surveillance system, the possibility of silent atherosclerotic cardiovascular disease could not be ruled out. Novel clinical modalities for quantitative estimates of the severity of atherosclerosis need to be developed in the near future (14).
The research field of cardio-oncology has grown because of the substantial increase in cancer survivors suffering from subsequent cardiovascular complications (15), and a possible link of cardiovascular disease with cancer development has also now come into focus (4), (5), (16). Regarding the purpose of the present study to address the potential association of the severity of atherosclerosis (i.e., the presence of polyvascular disease) with cancer development, we need to consider an influence of various shared fields to develop atherosclerosis and cancer. In the present study, nearly half of the patients had a history of cigarette smoking, which is a well-recognized risk factor for both atherosclerotic cardiovascular disease and cancer (17), and the prevalence of smokers was higher in the cohort with polyvascular disease than that in the cohort with CAD only. Nevertheless, cigarette smoking was found to be a weak hazard risk for an incidence of cancer. As one of the possibilities, enforced cessation of cigarette smoking when enrolled in the SHIP surveillance system may have affected the present findings because the basic role of modification of habitual behaviors to prevent both atherosclerotic cardiovascular disease and cancer is well-established (17). In addition, there was no interaction effect of various clinical differences as to the presence of polyvascular disease on the incidence of cancer. The high incidence of cancer in the cohort with polyvascular disease was also relatively unchanged, irrespective of the presence or absence of invasive coronary therapies. According to the annual data of the National Cancer Center between 2016 and 2018 (18), a crude annual incidence of cancer in a cumulative total number of 291,116,000 Japanese at the ages of over 15 years was 1.0%, which was clearly low, as compared with 5.2% in the present study. These also proposed a possible impact of the presence of atherosclerosis on cancer development.
Why would the severity of atherosclerosis predispose to develop cancer? Atherosclerosis is well-recognized as a chronic condition of premature vascular aging with a growth of atherosclerotic plaques, pathologically showing a loss of elasticity due to a decrease in numbers of vascular smooth muscle cells and a compensatory increase in collagen fibers (19). The phenomenon of vascular cellular senescence due to chronological aging is generally considered a natural barrier to protect against genome instability, which is the hallmark of cancer development (20), but this phenomenon has another feature, which is to secrete pro-inflammatory factors, namely, senescence-associated secretory phenotypes, to promote various aspects of cancer in a non-autonomous manner, including cancer cell proliferation, migration, invasiveness, angiogenesis, and epithelial-mesenchymal transition (21). Furthermore, with inflammation as the pivotal element in the pathogenesis of both atherosclerosis and cancer (22), (23), (24), atherogenic inflammation (12) with stress-induced premature senescence-associated secretary phenotypes (25), (26) may synergistically provide the favorable platform to develop cancer. Interestingly, in the field of clinical onco-cardiology, a combination of subtle inflammation and cancer also showed a possible risk for recurrent atherosclerotic events after percutaneous coronary intervention for the treatment of CAD (27). Recently, the presence of age-related clonal hematopoiesis, the so-called clonal hematopoiesis of indeterminate potential, has also been proposed as one of the key pro-inflammatory drivers for a link between atherosclerotic cardiovascular disease and cancer (28), (29). To investigate the central role of atherosclerosis in cancer development, we are now exploring the analysis of common extracellular vesicles between atherosclerotic cardiovascular disease and cancer (30) using practical applications of liquid biopsy (31), (32).
The present study has several limitations. First, the possibility of undetected cancer during the follow-up periods could not be excluded in the present study. However, this probability seemed to be similar between the two cohorts with CAD only and polyvascular disease; thus, it may not have affected the reliability of the present findings. Second, there were no differences in the distribution of organ sites of cancer between the two cohorts, but the present SHIP surveillance system has limited ability to identify detailed information as to each stage of cancer and also cancer therapies. We would create a more comprehensive surveillance system to analyze the characteristics of cancer in patients with any cardiovascular diseases. Third, a well-matched propensity score analysis provided a result consistent with the crude analysis regarding the association between polyvascular disease and the incidence of cancer, but this analysis could not eliminate the effects of unobserved confounding variables such as family histories, alcohol consumptions, duration of cigarette smoking, and a presence of atherosclerotic cerebrovascular disease, etc. Even though the present study was carefully constructed in accordance with the “Strengthening the Reporting of Observational Studies” in Epidemiology guidance (33), the study results need to be confirmed by nationwide studies.
In the present study, an independent association of polyvascular disease with the incidence of cancer in patients with CAD was found, implying the possibility that the severity of atherosclerosis could play a pivotal role in cancer development.
None
This work was supported by the Sakakibara Clinical Research Grant for Promotion of Science in 2018 grant number [H-6-2018].
The authors are sincerely grateful to Mr. Yoshikazu Yaegashi, Mr. Toshiaki Masuda, Ms. Saori Naitou, and Ms. Ikuko Shinmura for their profound contributions in assisting with this study and the data management in the Sakakibara Health Integrative Profile (SHIP) surveillance system. The authors are also very grateful to Dr. Tatsuya Murai, a distinguished cardiovascular pathologist, for his suggestion regarding the pathology of atherosclerosis and all of the staff for their clinical efforts as the heart team of the Sakakibara Heart Institute.
Makoto Suzuki: conceptualization, formal analysis, and writing of the original draft; Hitonobu Tomoike: conceptualization, data curation, supervision, methodology, writing of the review, and editing; Zhehao Dai: formal analysis, writing of the review, and editing; Toru Hosoda: writing of the review and editing; Tetsuya Sumiyoshi: project administration, writing of the review and editing; Saichi Hosoda: resources, writing of the review, and editing; and Mitsuaki Isobe: supervision, writing of the review, and editing.
September 16th, 2003, in the Sakakibara Heart Institute (no. 11000304)
ClinicalTrials.gov. number: NCT04198896.
Mitsuaki Isobe is one of the Associate Editors of JMA Journal and on the journal’s Editorial Staff. He was not involved in the editorial evaluation or decision to accept this article for publication at all.
Dagenais GR, Leong DP, Rangarajan S, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020;395(10226):785-94.
Koene RJ, Prizment AE, Blaes A, et al. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104-14.
Hasin T, Iakobishvili Z, Weisz G. Associated risk of malignancy in patients with cardiovascular disease: evidence and possible mechanism. Am J Med. 2017;130(7):780-5.
Aboumsallem JP, Moslehi J, de Boer RA. Reverse cardio-oncology: cancer development in patients with cardiovascular disease. J Am Heart Assoc. 2020;9(2):e013754.
Moslehi J, Zhang Q, Moore KJ. Crosstalk between the heart and cancer: beyond drug toxicity. Circulation. 2020;142(7):684-7.
Suzuki M, Tomoike H, Sumiyoshi T, et al. Incidence of cancers in patients with atherosclerotic cardiovascular diseases. Int J Cardiol Heart Vasc. 2017;17:11-6.
Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180-9.
Gutierrez JA, Aday AW, Patel MR, et al. Polyvascular disease: reappraisal of the current clinical landscape. Circ Cardiovasc Interv. 2019;12(12):e007385.
Japanese Circulation Society [Internet]. JCS Guidelines. 2022. Available from: https://www.j-circ.or.jp/guideline/guideline-series/.
Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179-91.
Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135-43.
Subherwal S, Bhatt DL, Li S, et al. Polyvascular disease and long-term cardiovascular outcomes in older patients with non-ST-segment-elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2012;5(4):541-9.
MacRae CA, Califf RM. Reimagining what we measure in atherosclerosis-a "phenotype stack". Circ Res. 2020;126(9):1146-58.
Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375(15):1457-67.
Narayan V, Thompson EW, Demissei B, et al. Mechanistic biomarkers informative of both cancer and cardiovascular disease. J Am Coll Cardiol. 2020;75(21):2726-37.
Rasmussen-Torvik LJ, Shay CM, Abramson JG, et al. Ideal cardiovascular health is inversely associated with incident cancer: the atherosclerosis risk in communities study. Circulation. 2013;127(12):1270-5.
National Cancer Center Japan [Internet]. Cancer Statistics. July 2021. Available from: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html.
Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111(2):245-59.
Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018;128(4):1238-46.
Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223-33.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-74.
Held C, White HD, Stewart RAH, et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial. J Am Heart Assoc. 2017;6(10):e005077.
Huynh K. Inflammation: targeting inflammatory pathways to treat atherosclerosis and cancer. Nat Rev Cardiol. 2017;14(11):629.
Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100(1):15-26.
Childs BG, Baker DJ, Wijshake T, et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472-7.
Tabata N, Sueta D, Yamamoto E, et al. Outcome of current and history of cancer on the risk of cardiovascular events following percutaneous coronary intervention: a Kumamoto University Malignancy and Atherosclerosis (KUMA) study. Eur Heart J Qual Care Clin Outcomes. 2018;4(4):290-300.
Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111-21.
Libby P, Sidlow R, Lin AE, et al. Clonal Hematopoiesis: crossroads of aging, cardiovascular disease, and cancer. J Am Coll Cardiol. 2019;74(4):567-77.
Yoshikawa J, Suzuki M, Hosoda T, et al. Pathogenic mechanisms of cancer and cardiovascular diseases. ClinicalTrials.gov Identifier: NCT03051191 [Internet]. [cited 2022 Jul 7]. Available from: https://clinicaltrials.gov.
Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379(10):958-66.
Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018;379(18):1754-65.
Vandenbroucke JP, von Elm E, Altman DG et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18(6):805-35.