Corresponding author: Hidenori Onishi, o-hide68@u-fukui.ac.jp
DOI: 10.31662/jmaj.2023-0054
Received: April 4, 2023
Accepted: July 18, 2023
Advance Publication: September 27, 2023
Published: October 16, 2023
Cite this article as:
Kobuchi T, Onishi H, Yamamura O, Sakamaki I, Iwasaki H, Hayashi H. Frequency and Risk Factors for Persistent and Concomitant Symptoms after Hospital Discharge for Coronavirus Disease 2019 in the Pre-omicron Period: An Exploratory Longitudinal Study. JMA J. 2023;6(4):437-447.
Introduction: Many countries have reported persistent and concomitant symptoms of coronavirus disease 2019 (COVID-19). This study aimed to identify persistent COVID-19 and concomitant symptoms in discharged patients and identify the risk factors for such symptoms.
Methods: This study enrolled patients with COVID-19 who were admitted to the University of Fukui Hospital, Japan, and discharged between April 3, 2020, and August 19, 2021. Persistent and concomitant symptoms were confirmed based on medical examinations approximately 2 weeks after discharge. Patient characteristics and symptoms were collected from the patients’ medical records by a technical assistant.
Results: This study included 120 patients (60 men and 60 women; mean age, 53.5 ± 17.0 years). Persistent COVID-19 symptoms were observed in 62 patients (51.7%). The most common persistent symptom was weakened physical function, manifesting as physical weakness (48.4%) and muscle weakness (29.0%). Binary logistic regression analysis revealed that cough with expectoration within the acute phase of COVID-19 was a risk factor predisposing patients to COVID-19 sequelae (odds ratio: 2.94, 95% confidence interval: 1.300 - 6.630, p = 0.009).
Conclusions: The study findings suggest that productive cough in the acute phase is associated with subsequent physical and muscle weaknesses in the subacute phase.
Key words: Patient Discharge, Cough, Logistic Models, Japan, Coronavirus disease of 2019, Fatigue, Risk Factors, Muscle Weakness
Many countries have reported long-term health effects of coronavirus disease 2019 (COVID-19) (1), (2), (3). For example, a study in Japan reported persistent symptoms such as dyspnea, fatigue, cough, and dysosmia for >120 days after the COVID-19 onset (4). Old age, female sex, obesity, and presence of five or more symptoms in the acute phase of COVID-19 have been reported as risk factors associated with the persistent symptoms of COVID-19 (1). Furthermore, a Japanese investigation of COVID-19 sequelae in patients discharged after hospitalization between February 2020 and June 2020 identified sequelae in 48 of 63 enrolled patients (76%), with high rates of sequelae observed even among patients in their 20s (75%) and 30s (83%) (5). The definition of sequelae used in this previous investigation was determined according to symptomology that persisted for ≥14 days (5).
Nevertheless, the causes of persistent symptoms or sequelae in COVID-19 patients remain unclear, and treatment methods have yet to be established. Generally, patients affected by persistent symptoms or sequelae require assistance in returning to work, social life, and day-to-day life. In December 2021, Japan’s Ministry of Health, Labour, and Welfare issued guidelines termed “COVID-19 Medical Practice Guidelines, Supplementary Volume: Management of Post-COVID-19 Conditions (Provisional Version).” This document compiled information on best clinical practices concerning COVID-19 sequelae (6).
Fukui Prefecture has a population of approximately 760,000 and is located in central Japan, facing the Sea of Japan. The number of COVID-19 cases in this prefecture during the study period increased to 122 in the first period (March to June 2020), 122 in the second period (July to September 2020), 301 in the third period (October 2020 to February 2021), 876 in the fourth period (March to July 19, 2021), and 1,676 in the fifth period (March to July 19, 2021). Alpha- and delta-valiant strains were prevalent in the fourth and fifth trimesters (7). In Fukui Prefecture, ensuring that hospital beds are always available has facilitated a successful policy of early hospitalization for COVID-19 patients. Almost no patients have been forced to stay home and wait for a hospital bed after a COVID-19 diagnosis. This enabled early and timely treatment of COVID-19 and has thus more effectively prevented any adverse impacts due to delayed treatment. Analysis of the pathogenesis of COVID-19 in patients diagnosed in the current study will be useful for determining which medical activities to implement against COVID-19.
This study aimed to identify persistent and concomitant symptoms in patients admitted to the University of Fukui Hospital for COVID-19 based on a follow-up outpatient examination conducted 2 weeks after discharge. It also aimed to identify the same symptoms in the subacute phase in patients discharged from the hospital and to identify the risk factors for these symptoms. Furthermore, we present a brief literature review about the persistent symptomology of COVID-19, including studies of “long COVID” and “post-COVID-19.” This study was an exploratory investigation of the frequency of persistent symptoms in the subacute phase of COVID-19.
In this exploratory longitudinal study, we targeted consecutively presenting patients who were admitted to the University of Fukui Hospital for COVID-19 and discharged between April 3, 2020, and August 19, 2021. COVID-19 acceptance at the University of Fukui Hospital ranged from mild to moderate disease not requiring oxygen therapy. These patients then underwent a follow-up examination 2 weeks after discharge. During the study period, 151 COVID-19 patients were identified. Of these, 31 (30 patients who did not undergo the follow-up examination due to hospital transfer and one patient who did not meet the definition of patients without COVID-19 sequelae) were excluded from the current study, thus providing an analysis set of 120 patients (60 men and 60 women; mean age, 53.5 ± 17.0 years).
A technical assistant (a nurse) collected information on patient characteristics, date of COVID-19 onset, symptoms during the acute phase, and symptoms at the outpatient follow-up examination from medical records filed within the study period.
Persistent symptoms during the subacute phase of COVID-19 after hospital discharge were defined as symptoms that persisted for >14 days from the time of COVID-19 onset (5), (8). Furthermore, new concomitant symptoms that had appeared during the outpatient visit were included as persistent COVID-19 symptoms. At the time of the outpatient visit, any persistent, or concomitant symptom was considered a sequela of COVID-19.
This study was conducted with the approval of the ethics review committee and in accordance with the Declaration of Helsinki. All researchers involved in this study complied with the Ethical Guidelines for Medical and Biological Research Involving Human Subjects (MEXT/MHLW/METI Notification No. 1 of March 23, 2021).
Because this study only evaluated existing information (i.e., patient medical records) and did not collect new samples or data, neither written, nor oral consent was obtained from the study subjects. This process was formally waived by the ethics review board at our medical center. Information about the study was disclosed to study subjects via the study webpage (http://research.hosp.u-fukui.ac.jp/rinsho/ethicscommittee/koukai_list/#chiiki_iryou). The study subjects were allowed to refuse participation in the study or to withdraw their consent for participation at any time.
Statistical analysis was conducted using the EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan; ver.1.54) (9). EZR is a statistical software that extends the functionality of R and R commander (The R Project for Statistical Computing, Vienna, Austria) (9). Continuous variables such as age, body mass index, and duration of follow-up are expressed as means ± standard deviations. Nominal (categorical) variables are expressed as the number of cases and frequencies (%) for each item. The two groups were compared using the Mann-Whitney U test for continuous variables and the χ2 test (including Yates continuity correction) for nominal variables. The three or four groups were compared using the Kruskal-Wallis test (multiple comparisons of two groups at a time with post hoc adjustment and Steel-Dwass multiple comparisons) for continuous variables and Fisher’s exact test (multiple comparisons of two groups at a time with Bonferroni adjustment) for nominal variables. Multivariate binomial logistic regression analysis with the presence or absence of COVID-19 sequelae as a dependent factor was employed to detect risk factors for COVID-19 sequelae. Clinical characteristics and acute-phase symptoms at admission were used as independent variables. To complement the analysis of acute-phase symptoms, we considered sputum expectoration related to cough and pharyngeal pain. The presence of both expectoration and cough symptoms was considered one factor. Both expectoration and pharyngeal pain were also analyzed as one factor. Independent variables were selected as factors exhibiting statistical or marginal significance in univariate analysis. Furthermore, independent variables that influenced each other were avoided in the selection. Multivariate analysis No. 1 was conducted using expectoration, nasal congestion, pharyngeal pain, dysosmia, dysgeusia, age (years), and sex (men) as independent variables. Multivariate analysis No. 2 was conducted using cough with expectoration, nasal congestion, pharyngeal pain, dysosmia, dysgeusia, age (years), and sex (men) as independent variables. Multivariate analysis No. 3 was conducted using pharyngeal pain with expectoration, nasal congestion, dysosmia, dysgeusia, age (years), and sex (men) as independent variables. In all analyses, a two-sided p-value of <0.05 was considered the threshold for statistical significance, and a p-value range of 0.05 ≤ p < 0.1 was considered as marginally significant.
Clinical characteristics and changes in acute-phase symptoms at the time of admission are presented in Table 1. In addition, the trends of COVID-19 infection and vaccination status during the survey period in Fukui Prefecture are presented in Figure 1 (7), (10), (11). The most common acute symptom was pyrexia (90%), followed by cough (80.8%). Divided by the period of infection, the numbers of patients in this study were 28, 9, 3, 30, and 50 in the first, second, third, fourth, and fifth periods, respectively. In comparing the periods, there were significant changes in age (p < 0.001), pneumonia (p = 0.012), dysosmia (p = 0.034), dysgeusia (p = 0.023), and anorexia (p = 0.017). Also, smoking (p = 0.079), pharyngeal pain (p = 0.079), pharyngeal pain with expectoration (p = 0.065), myalgia (p = 0.059), and muscle weakness (p = 0.095) varied with marginal significance. Overall, only one patient was vaccinated against COVID-19.
Table 1. Clinical Characteristics and Changes in Acute-Phase Symptoms at the Time of Admission of COVID-19 Patients.
| Clinical characteristics | Total n = 120 |
1st period a n = 28 |
2nd period b n = 9 |
3rd period c n = 3 |
4th period d n = 30 |
5th period e n = 50 |
p-value |
|---|---|---|---|---|---|---|---|
| Age (years) | 53.5 ± 17.0 | 57.2 ± 14.1 | 74.7 ± 10.4 | 39.6 ± 19.8 | 52.1 ± 19.2 | 49.2 ± 14.8 | <0.001f |
| Sex (men) | 60 (50.0) | 14 (50.0) | 5 (55.5) | 1 (50.0) | 14 (46.6) | 26 (52.0) | 0.976 |
| Body mass index (BMI) (kg/m2) | 23.4 ± 4.0 | 23.7 ± 3.9 | 21.6 ± 3.4 | 21.8 ± 0.8 | 24.2 ± 4.0 | 23.2 ± 4.1 | 0.522 |
| BMI (unknown) | 2 (1.6) | - | - | - | 1(3.3) | 1(2.0) | - |
| Lifestyle habits | |||||||
| Smoking | 23 (19.6) | 3 (10.7) | 0 (0.0) | 1 (33.3) | 4 (13.3) | 15 (30.0) | 0.079 |
| Drinking alcohol | 49 (40.8) | 9 (32.1) | 3 (33.3) | 0 (0.0) | 13 (43.3) | 24 (48.0) | 0.435 |
| Underlying disease | |||||||
| Hypertension | 40 (33.3) | 14 (50.0) | 3 (33.3) | 1 (33.3) | 6 (20.0) | 16 (32.0) | 0.182 |
| Diabetes | 18 (15.0) | 5 (17.9) | 2 (22.2) | 0 (0.0) | 3 (10.0) | 8 (16.0) | 0.825 |
| Dyslipidemia | 20 (16.7) | 6 (21.4) | 1 (11.1) | 0 (0.0) | 4 (13.3) | 9 (18.0) | 0.816 |
| Hyperuricemia | 11 (9.2) | 3 (10.7) | 1 (11.1) | 0 (0.0) | 4 (13.3) | 3 (6.0) | 0.799 |
| Heart disease | 11 (9.2) | 1 (3.6) | 2 (22.2) | 0 (0.0) | 2 (6.7) | 6 (12.0) | 0.419 |
| Malignancy | 9 (7.5) | 3 (10.7) | 0 (0.0) | 1 (33.3) | 2 (6.7) | 3 (6.0) | 0.376 |
| Acute-phase symptoms | |||||||
| Cough | 97 (80.8) | 22 (78.6) | 7 (77.8) | 2 (66.7) | 24 (80.0) | 42 (84.0) | 0.818 |
| Expectoration | 57 (47.5) | 11 (39.3) | 5 (55.6) | 2 (66.7) | 12 (40.0) | 27 (54.0) | 0.574 |
| Pharyngeal pain | 61 (50.8) | 8 (28.6) | 6 (66.7) | 2 (66.7) | 16 (53.3) | 29 (58.0) | 0.079 |
| Cough with expectoration | 52 (43.3) | 9 (32.1) | 5 (55.6) | 1 (33.3) | 11 (36.7) | 26 (52.0) | 0.375 |
| Pharyngeal pain with expectoration | 35 (29.2) | 3 (10.7) | 4 (44.4) | 1 (33.3) | 8 (26.7) | 19 (38.0) | 0.065 |
| Respiratory discomfort | 23 (19.2) | 3 (10.7) | 3 (33.3) | 0 (0.0) | 7 (23.3) | 10 (20.0) | 0.490 |
| Pneumonia | 84 (70.0) | 23 (82.1) | 8 (88.9) | 0 (0.0) | 23 (76.7) | 30 (60.0) | 0.012g |
| Runny nose | 26 (21.7) | 6 (21.4) | 1 (11.1) | 2 (66.7) | 4 (13.3) | 13 (26.0) | 0.790 |
| Nasal congestion | 21 (17.5) | 2 (7.1) | 1 (11.1) | 2 (66.7) | 5 (16.7) | 11 (22.0) | 0.111 |
| Pyrexia | 108 (90.0) | 25 (89.3) | 7 (77.8) | 2 (66.7) | 4 (13.3) | 13 (26.0) | 0.122 |
| Headache | 38 (31.7) | 5 (17.9) | 3 (33.3) | 1 (33.3) | 11 (36.7) | 18 (36.0) | 0.442 |
| Dysosmia | 32 (26.7) | 12 (42.9) | 1 (11.1) | 1 (33.3) | 3 (10.0) | 15 (30.0) | 0.034h |
| Dysgeusia | 29 (21.7) | 11 (39.3) | 1 (11.1) | 1 (33.3) | 2 (6.7) | 14 (28.0) | 0.023i |
| Abnormal vision | 3 (2.5) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (2.0) | 1.000 |
| Arthralgia | 15 (12.5) | 4 (14.3) | 0 (0.0) | 0 (0.0) | 3 (10.0) | 8 (16.0) | 0.794 |
| Myalgia | 9 (7.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (20.0) | 3 (6.0) | 0.059 |
| Body pain | 22 (18.3) | 7 (25.0) | 1 (11.1) | 0 (0.0) | 5 (16.7) | 9 (18.0) | 0.879 |
| Muscle weakness | 3 (2.5) | 0 (0.0) | 1 (11.1) | 0 (0.0) | 2 (6.7) | 0 (0.0) | 0.095 |
| Physical weakness | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) | 1.000 |
| Thirst | 7 (5.8) | 4 (14.3) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 2 (4.0) | 0.424 |
| Diarrhea | 47 (39.2) | 10 (35.7) | 2 (22.2) | 1 (33.3) | 8 (26.7) | 26 (52.0) | 0.147 |
| Nausea | 4 (3.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (8.0) | 0.348 |
| Anorexia | 53 (44.2) | 6 (21.4) | 4 (44.4) | 0 (0.0) | 15 (50.0) | 28 (56.0) | 0.017j |
| Malaise | 70 (58.3) | 15 (53.6) | 6 (66.7) | 1 (33.3) | 17 (56.7) | 31 (62.0) | 0.821 |
| n (%), Mean ± standard deviation (unit) Fisher’s exact test was used for pairwise comparisons of categorical variables. The Kruskal-Wallis test was used for pairwise comparisons of continuous variables. The Bonferroni adjustment was used for multiple comparisons. f:a vs b p = 0.015, b vs d p = 0.031,b vs e p < 0.001; g:Fisher’s exact test revealed a significant difference overall, but multiple comparisons for each group using Bonferroni did not show a significant difference.; h:a vs d p = 0.064; i:a vs d p = 0.041;j:a vs e p = 0.042 |
|||||||
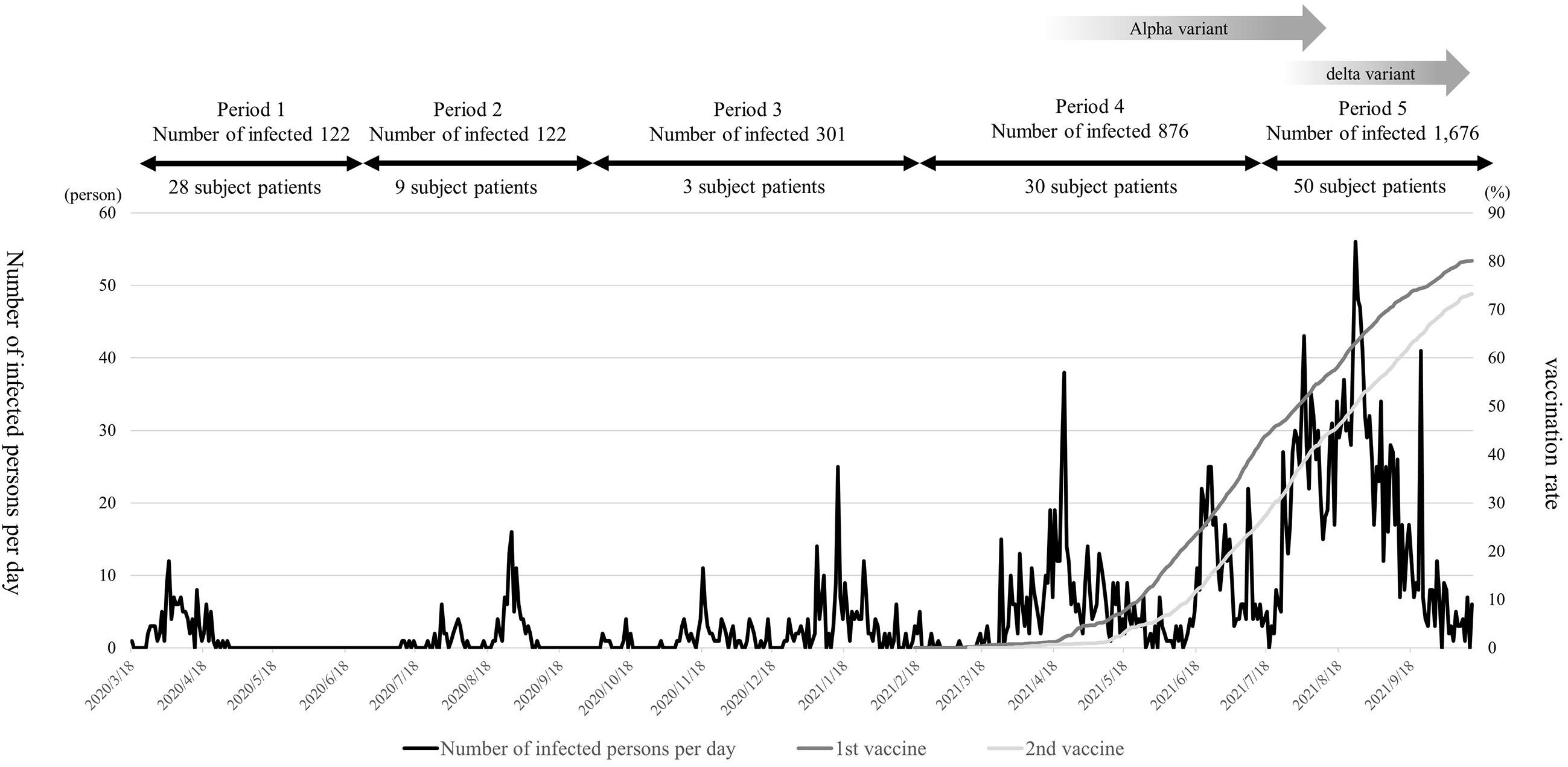
COVID-19 changes in symptoms and clinical characteristics of patients after discharge are presented in Table 2. The most common acute symptom was physical weakness (48.4%), followed by muscle weakness (29.0%). Divided by the period of infection, the numbers of patients in this study were 13, 5, 10, and 34 in the first, second, fourth, and fifth periods, respectively. In comparing the periods, significant changes were observed in smoking (p = 0.034).
Table 2. Clinical Characteristics in the Post-Discharge and Changes in the Timing of Concomitant Symptoms of COVID-19 Patients.
| Clinical characteristics | Total n = 62 |
1st period a n = 13 |
2nd period b n = 5 |
4th period c n = 10 |
5th period d n = 34 |
p-value |
|---|---|---|---|---|---|---|
| Age (years) | 53.7 ± 15.9 | 55.8 ± 15.5 | 71.0 ± 11.7 | 55.7 ± 20.1 | 49.7 ± 13.9 | 0.060 |
| Sex (men) | 30 (48.3) | 7 (53.8) | 3 (60.0) | 4 (40.0) | 16 (47.0) | 0.880 |
| Body mass index (BMI) (kg/m2) | 23.2 ± 4.3 | 24.0 ± 5.0 | 20.8 ± 3.4 | 23.9 ± 4.3 | 23.1 ± 4.2 | 0.566 |
| BMI (unknown) | 2 (3.2) | - | - | 1 (10) | 1 (2.9) | - |
| Lifestyle habits | ||||||
| Smoking | 12 (19.3) | 0 (0.0) | 0 (0.0) | 1 (10) | 11 (32.4) | 0.034e |
| Drinking alcohol | 26 (41.9) | 5 (38.5) | 2 (40.0) | 3 (30.0) | 16 (47.1) | 0.875 |
| Underlying disease | ||||||
| Hypertension | 19 (30.6) | 6 (46.2) | 1 (20.0) | 2 (20.0) | 10 (29.4) | 0.627 |
| Diabetes | 11 (17.7) | 2 (15.4) | 2 (40.0) | 1 (10.0) | 6 (17.6) | 0.584 |
| Dyslipidemia | 10 (16.1) | 1 (7.7) | 1 (20.0) | 1 (10.0) | 7 (20.6) | 0.680 |
| Hyperuricemia | 6 (9.6) | 1 (7.7) | 1 (20.0) | 1 (10.0) | 3 (8.8) | 0.826 |
| Heart disease | 8 (12.9) | 0 (0.0) | 2 (40.0) | 1 (10.0) | 5 (14.7) | 0.139 |
| Malignancy | 6 (9.6) | 2 (15.4) | 0 (0.0) | 2 (20.0) | 2 (5.9) | 0.349 |
| concomitant symptoms | ||||||
| Physical weakness | 30 (48.4) | 6 (46.2) | 4 (80.0) | 3 (30.0) | 17 (50.0) | 0.348 |
| Muscle weakness | 18 (29.0) | 4 (30.8) | 3 (60.0) | 3 (30.0) | 8 (23.5) | 0.384 |
| Cough | 10 (16.1) | 1 (7.7) | 0 (0.0) | 4 (40.0) | 5 (14.7) | 0.171 |
| Dysosmia | 9 (14.5) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 8 (23.5) | 0.247 |
| Dysgeusia | 9 (14.5) | 1 (7.7) | 1 (20.0) | 1 (10.0) | 6 (17.6) | 0.847 |
| Respiratory discomfort | 8 (12.9) | 0 (0.0) | 0 (0.0) | 3 (30.0) | 5 (14.7) | 0.159 |
| Malaise | 5 (8.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (14.7) | 0.362 |
| Sleep disturbance | 3 (4.8) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 2 (5.9) | 1.000 |
| Pyrexia | 2 (3.2) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (2.9) | 0.703 |
| Palpitation | 2 (3.2) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 1 (2.9) | 0.703 |
| Nasal congestion | 1 (1.6) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.452 |
| Headache | 1 (1.6) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.452 |
| Pharyngeal pain | 1 (1.6) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.452 |
| Chilblain | 1 (1.6) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.452 |
| n (%), Mean ± standard deviation (unit) Fisher’s exact test was used for pairwise comparisons of categorical variables. The Kruskal-Wallis test was used for pairwise comparisons of continuous variables. The Bonferroni adjustment was used for multiple comparisons. e:a vs b p = 0.015, b vs d p = 0.031,b vs e p < 0.001 |
||||||
A comparison of the presence of persistent and concurrent symptoms of COVID-19 after discharge is presented in Table 3. Persistent symptoms of COVID-19 were observed in 62 patients (mean age, 53.7 ± 16.0 years; 48.4% men), representing 51.7% of all patients enrolled in this investigation. The time from the onset of COVID-19 to the confirmation of COVID-19 sequelae in the subjects was 34.2 ± 8.7 days. Patients with persistent COVID-19 symptoms after discharge from the hospital had a significantly higher incidence of expectoration, cough with expectoration, and dysgeusia on admission (acute phase) compared with patients without persistent symptoms (expectoration: 59.3% vs 36.1%, p = 0.0179; cough with expectoration: 66.0% vs 29.3%, p = 0.00146; dysgeusia: 32.3% vs 15.5%, p = 0.0254). Marginal significance also included differences in pharyngeal pain with expectoration, nasal congestion, myalgia, and dysosmia (pharyngeal pain: 59.7% vs 41.4%, p = 0.068; pain with expectoration: 40.4% vs 22.0%, p = 0.075; nasal congestion: 24.2% vs 10.3%, p = 0.079; dysosmia: 37.1% vs 15.5%, p = 0.053).
Table 3. Comparison of COVID-19 Patients with and without Concurrent Symptoms after Discharge from Hospital.
| Overall n = 120 |
Patients with COVID-19 sequelae n = 62 |
Patients without COVID-19 sequelae n = 58 |
p-value | |
|---|---|---|---|---|
| Age, years n (%) | 53.5 ± 17.0 | 53.7 ± 16.0 | 53.3 ± 18.2 | 0.858 |
| Sex, men n (%) | 60 (50.0) | 30 (48.4) | 30 (51.7) | 0.855 |
| Body mass index (BMI) (kg/m2) | 23.4 ± 4.0 | 23.3 ± 4.4 | 23.6 ± 3.6 | 0.183 |
| BMI, unknown n (%) | 2 (1.6) | - | - | - |
| Lifestyle habits | ||||
| Smoking n (%) | 23 (19.6) | 12 (19.4) | 11 (19.0) | 1 |
| Drinking alcohol n (%) | 49 (40.8) | 26 (41.9) | 23 (39.7) | 0.946 |
| Underlying disease | ||||
| Hypertension n (%) | 40 (33.3) | 19 (30.6) | 21 (36.2) | 0.651 |
| Diabetes n (%) | 18 (15.0) | 11 (17.7) | 7 (12.1) | 0.539 |
| Dyslipidemia n (%) | 20 (16.7) | 10 (16.1) | 10 (17.2) | 1 |
| Hyperuricemia n (%) | 11 (9.2) | 6 (9.7) | 5 (8.6) | 1 |
| Heart disease n (%) | 11 (9.2) | 8 (12.9) | 3 (5.2) | 0.25 |
| Malignancy n (%) | 9 (7.5) | 5 (8.5) | 4 (6.6) | 0.959 |
| Acute-phase symptoms | ||||
| Cough n (%) | 97 (80.8) | 54 (87.1) | 43 (74.1) | 0.116 |
| Expectoration n (%) | 57 (47.5) | 35 (59.3) | 22 (36.1) | 0.0179 |
| Pharyngeal pain n (%) | 61 (50.8) | 37 (59.7) | 24 (41.4) | 0.0686 |
| Cough with expectoration n (%) | 52 (43.3) | 36 (66.0) | 16 (29.3) | 0.00146 |
| Pharyngeal pain with expectoration n (%) | 35 (29.2) | 23 (40.4) | 12 (22.0) | 0.0759 |
| Pneumonia n (%) | 84 (70.0) | 44 (71.0) | 40 (69.0) | 0.968 |
| Runny nose n (%) | 26 (21.7) | 16 (25.8) | 10 (17.2) | 0.359 |
| Nasal congestion n (%) | 21 (17.5) | 15 (24.2) | 6 (10.3) | 0.0793 |
| Pyrexia n (%) | 108 (90.0) | 59 (95.2) | 49 (84.5) | 0.1 |
| Headache n (%) | 38 (31.7) | 24 (38.7) | 14 (24.1) | 0.129 |
| Dysosmia n (%) | 32 (26.7) | 23 (37.1) | 9 (15.5) | 0.0539 |
| Dysgeusia n (%) | 29 (21.7) | 20 (32.3) | 9 (15.5) | 0.0254 |
| Abnormal vision n (%) | 3 (2.5) | 2 (3.2) | 1 (1.7) | 0.1 |
| Arthralgia n (%) | 15 (12.5) | 9 (14.5) | 6 (10.3) | 0.679 |
| Myalgia n (%) | 9 (7.5) | 5 (8.1) | 4 (6.9) | 1 |
| Body pain n (%) | 22 (18.3) | 14 (22.6) | 8 (13.8) | 0.314 |
| Muscle weakness n (%) | 3 (2.5) | 1 (1.6) | 2 (3.4) | 0.953 |
| Physical weakness n (%) | 1 (0.8) | 1 (1.6) | 0 (0) | 1 |
| Thirst n (%) | 7 (5.8) | 5 (8.5) | 2 (3.3) | 0.491 |
| Diarrhea n (%) | 47 (39.2) | 28 (45.2) | 19 (32.8) | 0.229 |
| Nausea n (%) | 4 (3.3) | 3 (4.8) | 1 (1.7) | 0.659 |
| Anorexia n (%) | 53 (44.2) | 29 (46.8) | 24 (41.4) | 0.681 |
| Malaise n (%) | 70 (58.3) | 41 (66.1) | 29 (50.8) | 0.108 |
| n (%), mean ± standard deviation (unit) Continuous variables: Mann-Whitney U test, Nominal variables: 2 test (including Yates continuity correction) |
||||
Risk Factors Associated with COVID-19 Sequela is presented in Table 4.
Table 4. Risk Factors Associated with COVID-19 Persistent and Concomitant Symptoms.
| Multivariate analysis No.1 | Multivariate analysis No.2 | Multivariate analysis No.3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI lower-upper |
p-value | OR | 95% CI lower-upper |
p-value | OR | 95% CI lower-upper |
p-value | |||
| Expectoration | 2.36 | 1.070-5.190 | 0.033 | Cough with expectoration | 2.94 | 1.300-6.630 | 0.009 | Pharyngeal pain with expectoration | 2.20 | 0.926-5.200 | 0.074 |
| Nasal congestion | 2.00 | 0.611-6.510 | 0.252 | Nasal congestion | 2.00 | 0.607-6.590 | 0.254 | Nasal congestion | 2.32 | 0.720-7.450 | 0.159 |
| Pharyngeal pain | 1.79 | 0.809-3.980 | 0.151 | Pharyngeal pain | 1.68 | 0.750-3.780 | 0.207 | Dysosmia | 2.36 | 0.760-7.310 | 0.137 |
| Dysosmia | 2.47 | 0.778-7.820 | 0.125 | Dysosmia | 2.23 | 0.698-7.140 | 0.176 | Dysgeusia | 1.75 | 0.566-5.380 | 0.332 |
| Dysgeusia | 1.60 | 0.509-5.030 | 0.422 | Dysgeusia | 1.66 | 0.520-5.300 | 0.392 | Age (years) | 1.02 | 0.991-1.040 | 0.212 |
| Age (years) | 1.01 | 0.989-1.040 | 0.255 | Age (years) | 1.01 | 0.987-1.040 | 0.333 | Sex (men) | 1.18 | 0.573-2.600 | 0.6770 |
| Sex (men) | 1.10 | 0.493-2.480 | 0.807 | Sex (men) | 1.05 | 0.464-2.380 | 0.905 | - | - | - | - |
| Multiple logistic regression analysis (binomial logistic regression analysis); CI: confidence interval | |||||||||||
Binomial logistic regression analysis revealed expectoration in the acute phase (odds ratio [OR] 2.36, 95% confidence interval [CI]: 1.070-5.190, p = 0.033) as a risk factor predisposing patients to COVID-19 sequelae.
Binomial logistic regression analysis revealed cough with expectoration in the acute phase (odds ratio [OR] 2.96, 95% CI: 1.300-6.630, p = 0.0094) as a risk factor predisposing patients to COVID-19 sequelae.
Binomial logistic regression analysis failed to indicate a factor as an independent variable.
We found a total of four articles that reported on the frequency of persistent symptoms and associated risk factors (1), (12), (13), (14) and nine articles that reported on the frequency of persistent symptoms only (2), (4), (5), (9), (15), (16), (17), (18), (19), (20). Representative data from these 12 studies as well as the present study are presented in Table 5.
Table 5. Frequency of Persistent Symptoms, Risk Factors, and Past Reports in COVID-19 Patients.
| This study and references | Country where the research was conducted | Study period | Target group | Number of subjects | Sex (Men) |
Age (years) |
Duration studied (after onset) |
Frequency | Most common symptom | Second most common symptom | Risk factor(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Japan | April 2020-August 2021 | Hospitalized | 120 | 50% | 53.5 ± 17.0 | Mean, 34.2 days | 51.70% | Physical weakness: 48.4% | Muscle weakness: 29.0% | Expectoration with cough (in the acute phase) |
| Tokyo iCDC Expert Board Infectious Disease Care Team (5) | Japan | Februar-June 2020 | Hospitalized | 63 | 66.7% | 48.1 ± 18.5 | 14 days | 76% | non | non | non |
| Miyazato et al. (4) | Japan | February-June 2020 | Hospitalized | 63 | 66.7% | 48.1 ± 18.5 | After 60 days | non | Dysosmia: 19% | Reparatory discomfort: 18% | non |
| After 120 days | non | Respiratory discomfort: 11% | Dysosmia: 10% Lassitude: 10% |
||||||||
| Petersen et al. (12) | Faroe Islands | April-June 2020 | Nonhospitalized | 180 | 46.0% | 39.9 (0-3) | Mean, 125 days | 53.0% | Malaise | Weakened sense of smell | non |
| Huang et al. (1) | China | Jan-May 2020 | Hospitalized | 1,733 | 52% | 57 (47-5) | After 6 months | 76% | Fatigue with muscle weakness: 63% | Sleep disturbance: 26% | Old age, female sex, obesity, 5 or more symptoms in the acute phase |
| Taquet et al. (16) | United States of America | July-December 2020 | COVID-19 infected | 273,618 | 44.4% | 46.3 ± 19.8 | 1-180 days | 57% (including acute-phase symptoms) |
Anxiety/depression: 22.8% | Abnormal respiration: 18.7% | Severe COVID-19, female sex, young adult |
| 90-80 days | 36.5% | Anxiety/depression: 15.5% | Abdominal symptom: 8.3% | ||||||||
| Logue et al. (13) | United States of America | August-November 2020 | Hospitalized (9.0%) Nonhospitalized (84.7%) |
177 | 42.9% | 48.0(18-4) | Mean, > 6 months | 31.1% | Malaise: 13.6% | Dysosmia/dysgeusia: 13.6% | non |
| Peghin et al. (18) | Italy | March-May 2020 | Hospitalized (26.2%) Nonhospitalized (73.7%) |
599 | 46.6% | 53 ± 15.8 | After 6 months | 40.2% (outpatients: 35.5%, inpatients: 53.5%) |
Malaise | Dysosmia/dysgeusia | Female sex, number of symptoms (proportional increase), admitted to the ICU |
| Nehme et al. (14) | Switzerland | March-May 2020 | Nonhospitalized | 410 | 32.9% | 42.7 ± 12.9 | After 7-9 months | 39.0% | Malaise: 20.7% | Dysosmia/dysgeusia: 16.8% | non |
| Liu et al. (2) | China | February-April 2020 | Hospitalized | 502 (traceable) |
46.3% (594 subjects) |
63 (53-68) (594 subjects) |
After 3 months | 51.2% | Insomnia: 16.9% | Tightness of the chest: 15.3% | non |
| 422 (Traceable) |
After 6 months | 40.0% | Chest tightness: 12.6% | Malaise: 6.4% | |||||||
| Huang et al. (17) | China | Jan- May 2020 | Hospitalized | 1,276 | 53% | 59(49-7) | After 6 months | 68% | Malaise or muscle weakness: 52.0% | Sleep disturbance:27.0% | non |
| After 12 months | 49% | Malaise or muscle weakness: 20.0% | Sleep disturbance:17.0% | ||||||||
| Asadi-Pooya et al. (20) | Iran | February 2020-March 2021 | Hospitalized | 2,685 | non | non | 3-6 months | 66% | Fatigue: 32% | Exercise intolerance: 26% | non |
| 1,996 | 6-12 months | 57.1% | Fatigue: 25% | Exercise intolerance: 20% |
This study focused on standard literature search databases such as PubMed, which were used to find studies on the frequency of persistent symptoms of COVID-19 (such symptomology is sometimes termed “long COVID” or “post-COVID syndrome”), examined the frequency of these persistent symptoms as well as their associated risk factors, and compared our findings with the results of existing reports.
More than half of the patients in this study had persistent and concomitant symptoms. The most common of these persistent and concomitant symptoms was a decline in physical function, with physical, and muscle weaknesses being the most common findings. Based on the study duration, it is reasonable to conclude that these persistent symptoms commonly occur in the subacute phase with COVID-19 sequela (persistent and concomitant symptoms).
COVID-19 is often compared with influenza, a respiratory infection lasting 2-8 days and causing a range of symptoms such as cough, pyrexia, myalgia, chills, sweating, and malaise (21). In this study, symptoms lasting 34.2 ± 8.7 days were observed in at least half of the enrolled patients, indicating that COVID-19 symptoms may persist for a longer period than the influenza symptoms.
In existing reports on persistent COVID-19 symptoms, the proportion of patients with persistent symptoms at 14 days from the time of disease onset (the shortest duration studied) was 76%, and the proportion of patients with persistent symptoms at 12 months from disease onset (the longest duration studied) was 49% (5), (17). Differences in study duration must be considered when comparing the frequency of persistent symptoms across studies, as must differences in subject characteristics. For example, studies that collected data after 6 months found that the proportion of patients with persistent symptoms widely ranged from 40.0% to 76.0% (1), (2), (17), (18). This variability may be caused by the different severities of COVID-19 and the different times of hospital admission among the studied subjects and may also be influenced by how each study defines “persistent symptoms” of COVID-19.
The World Health Organization (WHO) published a definition of “post-COVID-19 condition” on October 6, 2021, as follows (22): “post-COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually three months from the onset of COVID-19 with symptoms that last for at least two months and cannot be explained by an alternative diagnosis (22).” One of the reasons why studies published to date have examined different periods and have applied different definitions of persistent symptoms is that those investigations were conducted before the WHO published their definition.
Huang et al. found that the more severe the illness during the acute phase, the greater the proportion of patients with impaired respiratory function at a later date (1). The severity of COVID-19 in the acute phase among the studied subjects must also be considered. A major difference between this study and the previous ones is that delayed treatment had no impact on the persistent symptoms of COVID-19 in our study. In Fukui Prefecture, all people who have confirmed positive findings for SARS-CoV-2 must be hospitalized as a precaution against the spread of infection. Hence, all patients enrolled in this study were hospitalized early in their disease course. Furthermore, because cooperation between hospitals, and the local government in this prefecture ensures that hospital beds are always available, all patients receive early essential treatment for COVID-19. Nevertheless, even with early intervention, persistent symptoms of COVID-19 were observed in a high proportion of patients (51.7%).
Two multivariate analyses were conducted in this study. Expectoration (OR: 2.36) was a risk factor in the first analysis, and cough with expectoration (OR: 2.96) was a risk factor in the second analysis. Because the OR was higher in cases with expectoration and cough than with expectoration alone, we assume that cases with expectoration and cough are more important. In this study, we found that cough with expectoration in the acute phase of COVID-19 is a risk factor for persistent and concomitant symptoms occurring in the subacute phase after hospital discharge. SARS-CoV-2 proliferates in both the upper and lower respiratory tract, with particularly high viral loads observed in the upper respiratory tract (23). The main symptoms of COVID-19 are pyrexia and respiratory symptoms (e.g., cough) (24). Coughing is a protective reflex intended to remove foreign substances and airway secretions from the airways and is an important biological response (25). A previous study that investigated energy expenditure due to coughing showed an increase in energy expenditure of 11% from 2.72 ± 0.60 mL/kg/min at rest to 3.03 ± 0.62 mL/kg/min while coughing (26). Increased coughing due to respiratory infection also increases energy expenditure, which is very draining on the body. Furthermore, when coughing continues through the night, it can lead to sleep deprivation and delayed recovery. More than 70% of persistent symptoms observed in this study were manifestations of weakened physical function (physical and muscle weaknesses), indicating a possible relationship between persistent symptoms and physical exhaustion due to coughing and expectoration during the acute phase of COVID-19. In addition, although this study did not assess psychological effects, the long-term psychological impact of stress arising from disease-associated anxiety as well as the restrictions on movement associated with COVID-19 must be considered in interpreting our findings.
To the best of our knowledge, no established methods are available for the treatment of COVID-19 sequelae, and treatment instead mainly focuses on symptom management (6). The UK Health Security Agency has reported that people who have received one or more doses of a COVID-19 vaccine are less likely to develop Long-term effects of COVID-19 (long COVID) or post-COVID-19 syndrome than those who are unvaccinated (27). Only one patient in this study was vaccinated for COVID-19. If vaccinated patients had been included in this study, the frequency of persistent symptoms may have been lower. Furthermore, if COVID-19 vaccines are effective at preventing COVID-19 sequelae within rigorous, highly powered research, this would add to the evidence base supporting the importance of vaccines.
COVID-19 symptoms are thought to vary with the emergence of new variants and the status of immunity from vaccines and other sources (28). Also, the sequelae, and long-term manifestations of COVID-19 differ in symptoms and frequency of manifestations by the SARS-CoV-2 variants (29). The COVID-19 strains in this study include the conventional strain, α variant, and δ variant. In this study, the differences in symptoms during the acute phase were more pronounced in pneumonia, olfactory and taste disorders, and anorexia. However, the results are considered as reference information due to the small number of subjects in the second and third phases. There were no differences in persistent or concomitant symptoms at each time point. The number of patients in each study period does not allow for a clear comparison of symptoms.
This study had some limitations. First, for the explanation of the association between cough with expectoration in the acute phase and COVID-19 sequelae, we cannot estimate the exact causality. However, cough with expectoration in the acute-phase disability may be the reason for future COVID-19 sequelae after discharge. Second, this was a single-center study with a small sample size. Third, these findings may not be limited to COVID-19. Fourth, this study may have suppressed certain information due to interviewer bias. Fifth, physical information is not available in the form of body or muscle strength measurements. Sixth, the COVID-19 mutant strain of the subjects was unknown. The sixth aforementioned limitations should be considered in future studies.
In conclusion, we observed persistent, and concomitant symptoms of COVID-19 in the subacute phase in 51.7% of patients at hospital discharge. These symptoms include physical and muscle weaknesses, suggesting that persistent, and concomitant symptoms of COVID-19 were caused by physical fatigue induced by coughing. When comparing our findings with those of previous studies, differences in how persistent COVID-19 symptoms are defined must be considered. Our findings inform future research directions and directly inform medical guidelines and clinical decision-making.
None
We thank Editage (www.editage.com) for writing support.
All authors meet the ICMJE authorship criteria and contributed to the intellectual content of the manuscript. TK and HO designed the study and wrote the first draft of the manuscript. TK, HO, and OY were responsible for the data analysis. HH was responsible for the organization and coordination of the study. OY, IS, HI, and HH assisted in revising the manuscript. All authors are responsible for the interpretation of data and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors critically revised the manuscript for important intellectual content and approved the final version.
All data underlying the findings are within the paper.
This study was conducted with the approval of the University of Fukui Medical Research Ethics Review Committee (Approval No.: 20210098). This study was conducted in accordance with the Declaration of Helsinki. All researchers involved in this study complied with Ethical Guidelines for Medical and Biological Research Involving Human Subjects (MEXT/MHLW/METI Notification No. 1 of March 23, 2021). Because this study only evaluated existing information (i.e., patient medical records) and did not collect new samples or data, neither written nor oral consent was obtained from the study subjects. The study subjects were allowed to refuse participation in the study or to withdraw their consent for participation at any time. This process was formally waived by the ethics review board at our medical center. Information about the study was disclosed to study subjects via the study webpage (http://research.hosp.u-fukui.ac.jp/rinsho/ethicscommittee/koukai_list/#chiiki_iryou).
Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-32.
Liu T, Wu D, Yan W, et al. Twelve-month systemic consequences of coronavirus disease 2019 (COVID-19) in patients discharged from hospital: a prospective cohort study in Wuhan, China. Clin Infect Dis. 2022;74(11):1953-65.
Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine. 2021;38:1-19.
Miyazato Y, Morioka S, Tsuzuki S, et al. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis. 2020;7(11):ofaa507.
Tokyo center for infectious disease control and prevention (Tokyo iCDC). Expert Board, Infectious Disease Medical Treatment Team. COVID-19: registry-based research/epidemiological studies of sequelae. Disease Control and Prevention Center [Internet]. [cited 2021 Aug 29]. Available from: https://www.bousai.metro.tokyo.lg.jp/_res/projects/default_project/_page_/001/012/970/31kai/2021020407.pdf. Japanese.
Ministry of Health, Labour and Welfare. COVID-19 medical practice guidelines. Supplementary volume: management of post COVID-19 conditions (provisional version) [Internet]. [cited 2021 Dec 1]. Available from: https://www.mhlw.go.jp/content/000860932.pdf. Japanese.
Fukui prefectural. About looking of a for the 4th period 5th period of prefecture and future’s direction [Internet]. [cited 2023 May 18]. Available from: https://www.pref.fukui.lg.jp/doc/kenkou/corona/jyoukyou_d/fil/211111.pdf. Japanese.
Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993-8.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452-8.
Digital Agency. Vaccination record system (VRS) open data [Internet]. [cited 2023 May 18]. Available from: https://info.vrs.digital.go.jp/dashboard/. Japanese.
Fukui prefectural. Open data of the COVID-19 infection is exhibited [Internet]. [cited 2023 May 18]. Available from: https://www.pref.fukui.lg.jp/doc/toukei-jouhou/covid-19.html.
Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands: a longitudinal study among non-hospitalized patients. Clin Infect Dis. 2021;73(11):e4058-63.
Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830.
Nehme M, Braillard O, Chappuis F, et al. Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med. 2021;174(9):1252-60.
Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-5.
Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLOS Med. 2021;18(9):e1003773.
Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747-58.
Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27(10):1507-13.
Naik S, Haldar SN, Soneja M, et al. Post COVID-19 sequelae: a prospective observational study from Northern India. Drug Discov Ther. 2021;15(5):254-60.
Asadi-Pooya AA, Akbari A, Emami A, et al. Risk factors associated with long COVID syndrome: a retrospective study. Iran J Med Sci. 2021;46(6):428-36.
Gaitonde DY, Moore FC, Morgan MK. Influenza: diagnosis and treatment. Am Fam Physician. 2019;100(12):751-8.
A clinical case definition of post COVID-19 condition by a Delphi consensus World Health Organ. [Internet]. 2021. [cited 2022 Jan 29] Available from: https://apps.who.int/iris/bitstream/handle/10665/345824/WHO-2019-nCoV-Post-COVID-19-condition-Clinical-case-definition-2021.1-eng.pdf?sequence=1&isAllowed=y.
Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-9.
Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. NEJM. 2020;382(18):1708-20.
Fontana GA, Pantaleo T, Lavorini F, et al. A noninvasive electromyographic study on threshold and intensity of cough in humans. Eur Respir J. 1997;10(5):983-9.
Pontifex E, Williams MT, Lum R, et al. The effect of huffing and directed coughing on energy expenditure in young asymptomatic subjects. Aust J Physiother. 2002;48(3):209-13.
UK Health Security Agency. UKHSA review shows vaccinated less likely to have long COVID than unvaccinated [Internet]. [cited 2022 Feb 18]. Available from: https://www.gov.uk/government/news/ukhsa-review-shows-vaccinated-less-likely-to-have-long-covid-than-unvaccinated.
Looi MK. How are COVID-19 symptoms changing? BMJ. 2023;380:3.
Du M, Ma Yirui, Deng J, et al. Comparison of long COVID-19 caused by different SARS-CoV-2 strains: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(23):16010.