Corresponding author: Shugo Tohyama, shugotohyama@keio.jp
DOI: 10.31662/jmaj.2020-0036
Received: May 7, 2020
Accepted: May 18, 2020
Advance Publication: July 13, 2020
Published: July 15, 2020
Cite this article as:
Morita Y, Tohyama S. Metabolic Regulation of Cardiac Differentiation and Maturation in Pluripotent Stem Cells: A Lesson from Heart Development. JMA J. 2020;3(3):193-200.
The heart, one of the more complex organs, is composed from a number of differentiated cells. In general, researchers consider that the cardiac cells are derived from the same origin as mesodermal cells, except neural crest cells. However, as the developmental stages proceed, cardiac mesodermal cells are differentiated into various types of cells via cardiac progenitors and demonstrate different programming in transcriptional network and epigenetic regulation in a spatiotemporal manner. In fact, the metabolic feature also changes dramatically during heart development and cardiac differentiation. Researchers reported that each type of cell exhibits different metabolic features that can be used to specifically identify them. Metabolism is a critical process for generating energy and biomass in all living cells and organisms and has been long regarded as a passenger, rather than an active driver, for intracellular status. However, recent studies revealed that metabolism influences self-renewal and cell fate specification via epigenetic changes directly or indirectly. Metabolism mirrors the physiological status of the cell and endogenous cellular activity; therefore, understanding the metabolic signature of each cell type serves as a guide for innovative methods of selecting and differentiating desired cell types. Stem cell biology and developmental biology hold great promise for cardiac regenerative therapy, for which, successful strategy depends on the precise translation of the philosophy of cardiac development in the early embryo to the cell production system. In this review, we focus on the metabolism during heart development and cardiac differentiation and discuss the next challenge to unlock the potential of cell biology for regenerative therapy based on metabolism.
Key words: Metabolism, Pluripotent Stem Cells, Cardiac differentiation, Heart Development, Regenerative Medicine
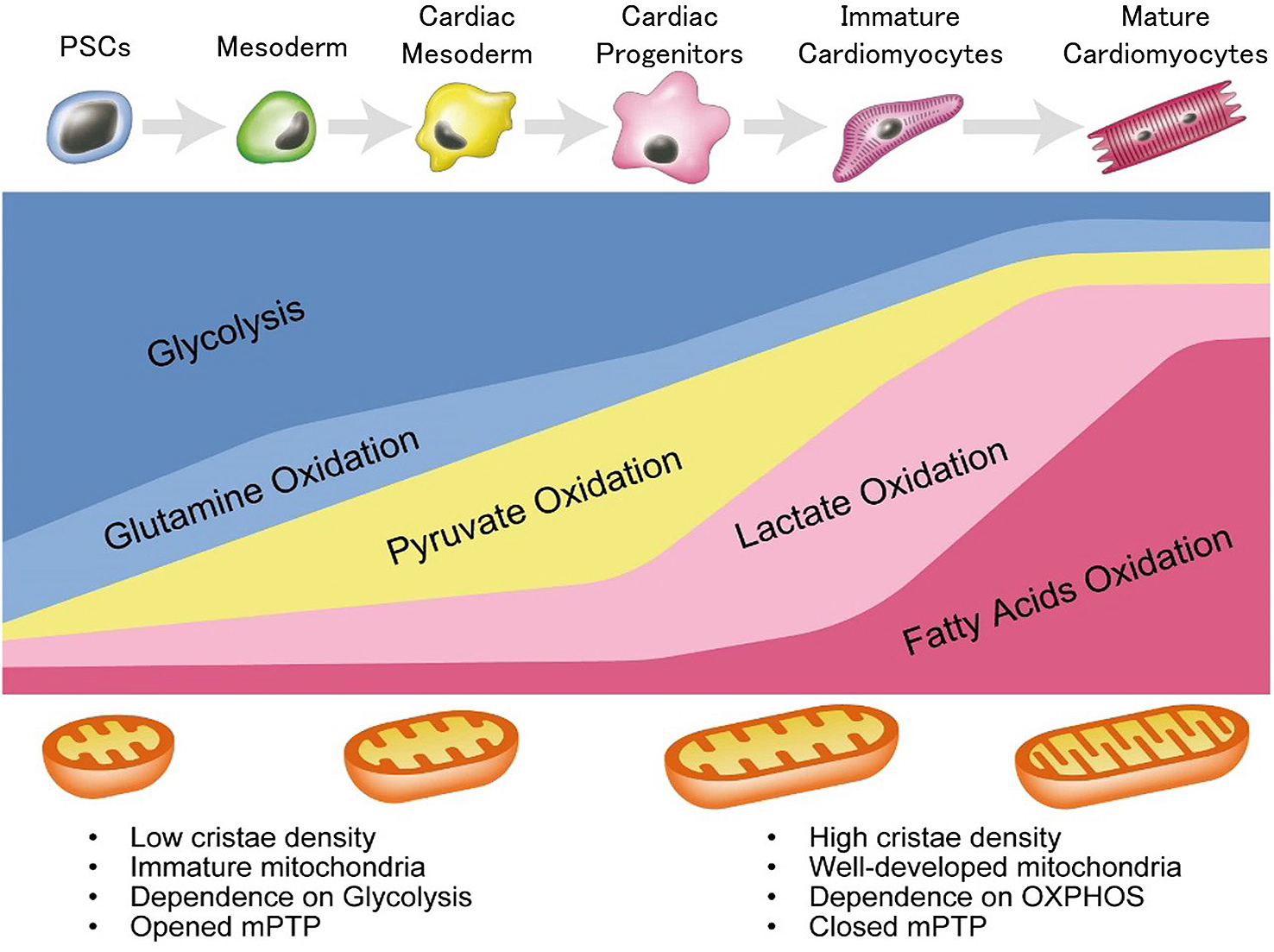
Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), are expected to be useful for cell-based therapy and pharmacological screening, owing to their ability for theoretically infinite production of required cell types including cardiomyocytes. For realization of cell-based therapy and pharmacological screening, pure and matured cardiomyocytes derived from human PSCs (hPSCs) are needed in large quantities. To obtain large quantities of pure and matured cardiomyocytes, development of efficient cardiac differentiation, purification, and maturation protocols is necessary. For the development of efficient cardiac differentiation protocols, complicated signaling in heart development was studied because processes of cardiac differentiation from PSCs are known to be similar to those of heart development (Figure 1) (1), (2), (3), (4). In cardiomyocyte purification, combinations of fluorescence activated cell sorting (FACS) and genetic modification or the use of antibodies for cardiomyocyte-specific surface proteins (e.g. VCAM-1, SIRPA) are major strategies (5), (6). However, due to the use of FACS, these strategies are not suitable for large-scale production of cardiomyocytes. In cardiac maturation, physical interventions, including tissue engineering and electrical stimulation, are known to be effective (7), (8). Conversely, many studies recently demonstrated that metabolic regulation also affects the survival, maintenance, and differentiation of PSCs (9), (10). Also, our group developed a large-scale metabolic selection system for hPSC-derived cardiomyocytes, based on developmental metabolic profiles (11), (12), (13), (14). These studies suggest that a better understanding of developmental metabolism demonstrates the potential to provide an alternative solution for improvement of cardiac differentiation, purification, and maturation in PSCs (Figure 2). Cellular metabolism was recognized as a passenger for PSCs and differentiated cells for proliferation, self-renewal, and differentiation. However, this concept is changing in that cell metabolism is one of the drivers for cell fate decision (15). Additionally, understanding cell metabolism may provide new insights on the phenomena of proliferation, differentiation, and maturation. Here, we review the metabolic shift as a key driver during heart development and cardiac differentiation.
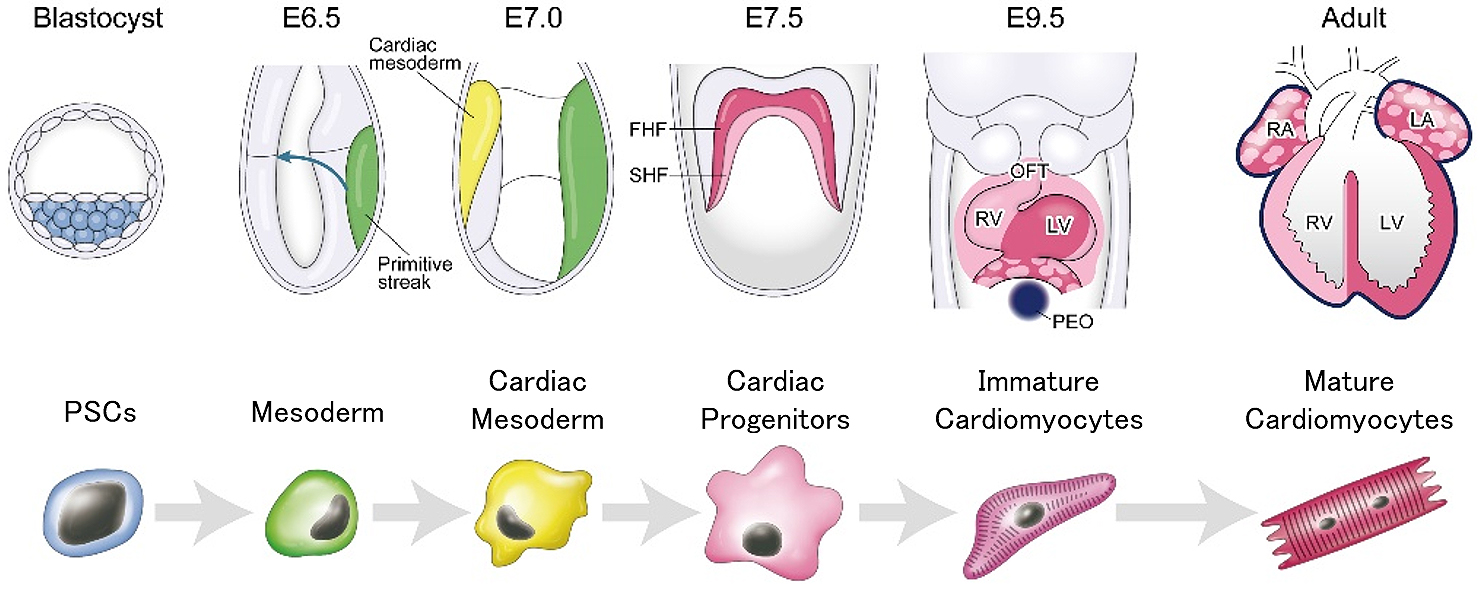
The blastocyst is composed of two distinct cell lineages: inner cell mass (ICM), which gives rise to definitive structures of fetus, and trophectoderm (TE), which predominantly forms extraembryonic lineages. The TE contains a greater number of mitochondria than ICM, consumes oxygen, and activates mitochondrial oxidative phosphorylation (OXPHOS) (16). Researchers considered that TE generates approximately 80% of ATP, which is mainly used for the sodium pump (Na+, K+, ATPase) located in the TE (16). In contrast, glycolysis is activated, and a lot of lactate is produced in ICM (Figure 1) (17), (18). ICM shows low mitochondrial membrane potential, indicating low levels of OXPHOS (19).
Amino acids are also known to be key molecules for proper blastocyst development (20). Interestingly, TE or ICM alone extracted from bovine blastocyst shows that amino acid turnover is dramatically increased. Furthermore, TE and ICM exhibit different amino acid profiles for consumption and production, respectively (21). In the bovine blastocyst, aspartate, glutamate, and arginine are consumed, while alanine is produced (21) Whereas, in ICM extracted from bovine blastocyst, aspartate, asparagine, glycine, threonine, arginine, tyrosine, tryptophan, leucine, and lysine are consumed, while glutamate, serine, histidine, glutamine, alanine, methionine, valine, and isoleucine are produced (21). In TE, leucine and arginine are preferably utilized in bovine, mice, and humans (21), (22), (23). Leucine and arginine alone are necessary and sufficient for mouse trophoblast outgrowth, and mTOR activation and protein translation are necessary for trophoblast outgrowth by these amino acids. This result suggests that leucine and arginine help in the attachment of trophectoderm to uterine epithelial cells to initiate the implantation (23). Besides, methionine metabolism is crucial for blastocyst development (24), (25). Methionine deprivation caused a reduced H3K4 methylation and altered gene expression profiles. Therefore, maternal methionine restriction induced embryonic lethality in mice (25).
Fatty acid metabolism also demonstrates a key role in blastocyst development. Octanoate, a medium-chain fatty acid, when incorporated into TCA cycle, produced ATP via β-oxidation process (26). Although blastocysts could not normally develop under culture conditions without fatty acids, glutamine, and pyruvate, octanoate supplementation can rescue blastocyst development, indicating that blastocysts utilize fatty acids for energy production, and octanoate can provide alternative energy source through preimplantation development (26).
As PSCs constantly self-renew and proliferate in vitro, protecting them from reactive oxygen species (ROS) via OXPHOS is crucial for maintaining genome stability because ROS induces nuclear and mitochondrial DNA damage, protein oxidation, and lipid oxidation. Recent studies showed that hPSCs exhibits different metabolic characters depending on pluripotency state, and these differences demonstrate an influence on distinct differentiation potential (27). Nevertheless, similar to embryo development, mouse and human PSCs mainly depend on glucose and glutamine metabolism (Figure 2) (12), (28). This preference makes sense in suppression of ROS generation for PSCs. Naïve mouse PSCs (mPSCs) utilize glucose and glutamine to maintain high α-ketoglutarate (αKG) levels because elevated αKG promotes histone/DNA demethylation and contributes to the maintenance of pluripotency (29). In addition, glucose and glutamine metabolism contributes to both ATP generation and biomass production in hPSCs (12), (29), (30). Interestingly, glycolytic genes precede pluripotent marker genes in the reprogramming process in mPSCs (30). Moreover, glucose-derived cytosolic acetyl-CoA promotes histone acetylation under pluripotent state in mouse and human PSCs (31). To keep pluripotency in mouse and human PSCs, histone acetylation also demonstrates a pivotal role in the maintenance of an open chromatin structure (32), (33), (34), (35). Furthermore, glutamine metabolism also contributes to maintain pluripotency because glutamine-derived reduced glutathione prevents degradation of OCT4. Among the other amino acids, threonine and methionine contributes to S-adenosylmethionine (SAM) production in mouse and human PSCs, respectively, leading to subsequent trimethylation of histone H3 lysine 4 (H3K4me3) for the maintenance of pluripotency (19), (36). Researchers also reported that de novo fatty acid synthesis regulated by lipogenic enzyme ACC1 promotes reprograming of human fibroblasts by affecting mitochondrial fission (37).
Since glucose and glutamine metabolism are essential for the maintenance of pluripotency in ICM and mouse and human PSCs, reduction of glucose and glutamine metabolism will affect the differentiation efficiency. Predictably, when mouse and human PSCs exit pluripotency, metabolism switches away from glycolysis (31), (35). In fact, during early differentiation from hPSCs, metabolic switching by MYC/MYCN is a critical step for germ layer specification. In hPSCs, stable expression of MYC/MYCN transcription is required for the maintenance of elevated glycolysis. In addition, each decline in MYC or MYCN expression is required for the transition of pluripotency to the mesoderm or definitive endoderm, resulting in metabolic switching from glycolysis to OXPHOS (Figure 2) (38). Furthermore, retinoic acid, an upstream negative regulator of MYC/MYCN, also induces differentiation from primed hPSCs, with decreased glucose and increased oxygen consumption (35). Mesodermal cells show a higher oxygen consumption rate (OCR) and lower extracellular acidification rate (ECAR) than hPSCs, ectoderm, and endoderm cells (39). Before differentiation, pyruvate supplementation, the main product of glycolysis upregulates the OCR/ECAR ratio and expression of mesodermal markers, and it induces mesodermal cell via activation of AMPK pathway (40). Researchers reported that insulin blocked mesendodermal differentiation and cardiogenesis from hPSCs by inhibiting the expression of Brachyury (T), Foxa2, Gata4, and Nkx2-5 (41), (42). Moreover, insulin is known to be associated with glycolysis. When insulin binds to its receptor in the cell membrane, it promotes recruitment of glucose transporters from cytoplasm to cell membrane, leading to increase in glucose uptake. This suggests that insulin depletion forces the metabolic changes from glycolysis to OXPHOS during cardiac differentiation. This metabolic change may be associated with the early mesodermal differentiation in mouse and human PSCs. In mesodermal induction, it is well known that the activation of canonical Wnt/β-catenin signaling is indispensable (3). Glycogen synthase kinase 3β (GSK3β) is involved in the phosphorylation of β-catenin, leading to degradation by proteasome. Therefore, GSK3β inhibitors (e.g. CHIR99021) activate Wnt/β-catenin signaling. Besides, GSK3 is originally known to affect glucose metabolism through insulin signaling (43). In short, GSK3 inhibits glycogen synthesis via phosphorylation of glycogen synthase (GS), which is a key enzyme to convert glucose to glycogen, inducing activated glycolysis.
The Hippo signaling pathway regulates the cell proliferation and organ size. Yes-associated protein (Yap) is one of the critical downstream effectors of the Hippo pathway, and it interacts with SMAD and Oct4. Yap-SMAD-Oct4 binds with NuRD repressor complex to maintain pluripotency and inhibit mesodermal differentiation of hPSCs via a decrease in Wnt3 expression (44), (45). In zebrafish liver and human liver cancer cells, Yap directly enhances glutamine synthetase expression and activates glutamine metabolism for nucleic acid synthesis (46). Furthermore, the regulation of glucose transporter 1 (Glut1) expression and glucose uptake by Yap is highly conserved in mammals (47). Therefore, reduction of Yap expression is essential for metabolic transition from glycolysis/glutamine metabolism to OXPHOS and important for mesodermal differentiation. Especially, most cancer cells mainly rely on the activated glycolysis via GSK3-mediated inhibition of GS even under normoxia, which is called the “Warburg Effect.” Hence, inhibition of activated glycolysis by restricting GSK3 function is considered a target of cancer therapeutics. Researchers also reported that GSK3 increased glycolytic flux to proliferate B cells when the antigen is encountered, indicating that GSK3 acts as a metabolic sensor to regulate immune response by regulating the glycolytic pathway (48). In skeletal muscle, inactivation of GSK3β increases fatty acid oxidation (FAO) via activation of PPAR-γ-co-activator1, a master regulator of mitochondrial biosynthesis (49). Taken together, the assumption exists that GSK3 inhibition induces a metabolic shift from glycolysis to OXPHOS during mesodermal differentiation.
Although a developing heart mainly depends on OXPHOS compared to ICM and PSCs, metabolic changes also occur during heart development. As the embryonic and fetal hearts exist in hypoxic environment, they depend on glycolysis rather than OXPHOS to generate ATP (Figure 2). Glucose dependence of embryonic and fetal heart may be due to hypoxia inducible factor 1α (HIF-1α), a transcriptional factor activated by low oxygen. HIF-1α, a key regulator for glycolysis, regulates glycolysis-related genes, such as glut1 and hexokinase1 (hk1), by directly binding to hypoxia response elements located on the upstream of HIF-1α target genes (50), (51). During heart development, Nkx2-5-specific deletion of HIF-1α causes hypoplasia and arrested cardiac development in mice (52). Moreover, α-myosin heavy chain (αMHC)Cre/+-VHL mice, which maintain stable expression of HIF-1α in normoxic condition, show low mitochondrial function and ATP generation, suggesting that the decreased HIF-1α signaling in cardiomyocytes after birth controls metabolic changes from glycolysis to OXPHOS (53). In fact, embryonic and fetal cardiomyocytes do not depend much on FAO under 15% of total ATP production (51). Conversely, a loss of mitochondrial transcription factor A (known as tfam) causes severe mtDNA depletion with abolished OXPHOS and leads to heart malformation and embryonic lethal prior to E10.5 in mice, suggesting that mitochondria also exhibit a pivotal role in heart development, because dependency on OXPHOS in embryonic and fetal hearts is still higher than that in ICM and early differentiated cells (54). Mitochondrial morphological changes are also important for cardiac differentiation in developing hearts (Figure 2). The mitochondrial permeability transition pore (mPTP) is open in embryonic hearts, while it is closed during development. Closed mPTP drives mitochondrial elongation and higher mitochondrial membrane potential in mouse hearts (55).
During cardiac differentiation from mPSCs, the basal mitochondrial respiration and respiratory capacity is significantly increased, whereas glycolysis is decreased (56). The expression levels of the genes involved in mitochondrial fusion and cristae maturation in differentiated cardiomyocytes are higher than those in mPSCs (56). Consistent with this, mouse and human PSC-derived cardiomyocytes show higher mitochondrial OXPHOS (Figure 2) (56). Inhibition of OXPHOS during cardiac differentiation causes reduction and abnormal translocation of mitochondria, leading to poor sarcomeric organization and a lower beating frequency (56). Moreover, as with a developing heart, mPTP inhibition by cyclosporine A also induces mitochondrial maturation and cardiac differentiation in mouse and human PSCs (57). These findings suggest that mitochondrial maturation and metabolic shift are necessary for cardiac differentiation from PSCs.
Even immediately after birth, cardiomyocytes still rely on glycolysis and lactate oxidation (LO) as a source of ATP production. On day 7 after birth, glycolysis-dependency decreases to less than 10 % of total ATP production accompanied with increased dependence on LO and FAO in rabbit neonatal hearts (Figure 2) (50). Researchers reported that this metabolic change and cell cycle arrest through DNA damage response were affected by oxygen-rich environment (58). The dependence on LO dramatically decreases to less than 1% and that on FAO increases up to 80% of total ATP production, due to increased uptake of fatty acids for FAO in 21 days old rabbit neonatal hearts (Figure 2) (50).
It has been known that hPSC-derived cardiomyocytes are much less organized and smaller than the adult cardiomyocytes and resemble fetal cardiomyocytes, as described previously (59), (60). The condition of cardiac maturation is evaluated by structure, gene expression, energy, force, conduct, ion channel density, and Ca2+ kinetics (59). Understanding of heart developmental metabolism enables us to develop a method for obtaining matured and pure cardiomyocytes from hPSCs. Although hPSCs and hPSC-derived non-cardiac proliferating cells mainly depend on glucose and glutamine metabolism, differentiated immature cardiomyocytes rely on LO for ATP production (Figure 2) (12). By utilizing these metabolic differences, we successfully selected only cardiomyocytes under glucose- and glutamine-depleted but lactate containing conditions. Remarkably, this method forces a metabolic shift from glycolysis to LO and makes sarcomeric organization and mitochondria developed (14). Furthermore, metabolically selected PSC-derived cardiomyocytes enhance the ratio of OCR/ECAR by 1.8-fold compared to non-purified cardiomyocytes (61), suggesting that this method forces the metabolic maturation from embryonic to neonatal cardiomyocytes. These metabolic shifts of cardiomyocytes result into more mature action potentials and calcium handling with increased expression of cardiomyocyte-related ion channels and cardiac contraction genes (62). Researchers reported that activated glycolysis inhibited cardiac maturation through pentose phosphate pathway, which supplies NADPH, nucleotides, and lipids for proliferation. In contrast, glucose-depleted conditions promoted cardiac maturation with the increased expression of cardiac contract genes, functional beating, and morphological maturity in vitro (63). In addition, the efficiency of cardiac differentiation from mESCs is better in low glucose medium than in high glucose medium as high glucose medium suppresses the expression of mesoderm and cardiac transcription genes, resulting in the reduction of contractile cardiomyocytes (64). As one of the positive factors for cardiac maturation, researchers reported that palmitate administration accelerates cardiac maturation with highly organized sarcomeric structure, mechanical force, and gene expression profiles in cardiac organoids derived from hPSCs (65). Similar to postnatal hearts, the metabolic switching from glycolysis to FAO induces cardiac maturation through DNA damage response and cell cycle arrest (Figure 2). Furthermore, PGC1α, a major regulator of mitochondria, also plays a key physiological role in cardiac maturation by controlling ROS (66). According to the assessment of oxidative metabolism in hPSC-derived cardiomyocytes cultured in glucose containing media, glucose and fatty acid containing media, and fatty acid containing media, the fatty acid containing media enhanced oxidative metabolism and cardiac maturation, structurally and functionally (67). Genome-wide effects of fatty acid treatment showed that the genes involved in fatty acid transportation were upregulated, whereas the genes involved in glucose metabolism were downregulated (68). Moreover, extracellular matrix, providing essential scaffold for intercellular contact and signal molecules, is also a key regulator for cardiac maturation. mESC-derived cardiomyocytes, cultured on laminin-511/521, showed structural maturation and increased mitochondrial respiration (69). These findings indicate that both the intracellular metabolic profiles and mitochondrial function are critical for cardiac maturation as well as cardiac differentiation (58).
Since the beginning of the learning of how to make a heart, researchers revealed that in addition to the network of transcriptional factors and epigenetic modifiers, metabolism is also dramatically changed in a spatiotemporal manner. The mechanisms of cardiac differentiation in vitro are highly correlated with the heart development in vivo; therefore, the methods of cardiac differentiation from PSCs are continuously improving in terms of differentiation efficiency and maturity. However, many issues such as variation among batches and lots are yet to be explored for an effective cardiac differentiation method. In addition, the same quality of differentiated cardiomyocytes should be obtained, physiologically and functionally. Furthermore, it is known that PSC-derived cardiomyocytes are more immature than adult cardiomyocytes in vivo, and a detailed understanding of the mechanisms for maturation of cardiomyocytes are also needed. In this review, we summarized the metabolic shift that characterized gene expression, structure, and function during cardiac differentiation. Comprehension among transcriptional network, signal pathway, and metabolism will give us breakthrough achievement. In the future, it may be realized that this knowledge on the metabolism in each cell type will give us better and correct understandings of the network of metabolic plasticity, gene regulation, and epigenetic modification. Furthermore, the metabolic feature may become an important criterion of differentiation, maturation, and disease state. If so, the metabolic information of the cells may be the factor to further ensure the safety of cell transplantation.
This article is based on the study, which received the Medical Research Encouragement Prize of The Japan Medical Association in 2019.
S. T. owns equity in Heartseed. Inc.
This work was supported by the Medical Research Encouragement Prize of The Japan Medical Association (S. T.) and Projects for Technological Development, Research Center Network for Realization of Regenerative Medicine by Japan, the Japan Agency for Medical Research and Development grant (20bm0404023h0003 to S. T.), and partly supported by JSPS KAKENHI grant (19K22626 to S. T, 20J01097 to Y. M.).
Y. M. and S. T. made substantial contributions to the conception and design of the work.
This manuscript is a review and therefore did not need approval by an IRB.
Yuasa S, Itabashi Y, Koshimizu U, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23(5):607-11.
Shimoji K, Yuasa S, Onizuka T, et al. G-CSF promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs. Cell Stem Cell. 2010;6(3):227-37.
Lian X, Zhang J, Azarin SM, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc. 2013;8(1):162-75.
Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8(2):228-40.
Uosaki H, Fukushima H, Takeuchi A, et al. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One. 2011;6(8):e23657.
Dubois NC, Craft AM, Sharma P, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29(11):1011-8.
Eng G, Lee BW, Protas L, et al. Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nat Commun. 2016;7(1):10312.
Nunes SS, Miklas JW, Liu J, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10(8):781-7.
Ben-David U, Gan QF, Golan-Lev T, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12(2):167-79.
Shiraki N, Shiraki Y, Tsuyama T, et al. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 2014;19(5):780-94.
Tohyama S, Hattori F, Sano M, et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12(1):127-37.
Tohyama S, Fujita J, Hishiki T, et al. Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metab. 2016;23(4):663-74.
Tohyama S, Fujita J, Fujita C, et al. Efficient large-scale 2D culture system for human induced pluripotent stem cells and differentiated cardiomyocytes. Stem Cell Rep. 2017;9(5):1406-14.
Tohyama S, Fukuda K. Safe and effective cardiac regenerative therapy with human-induced pluripotent stem cells: how should we prepare pure cardiac myocytes? Circ Res. 2017;120(10):1558-60.
Zhang J, Zhao J, Dahan P, et al. Metabolism in pluripotent stem cells and early mammalian development. Cell Metab. 2018;27(2):332-8.
Houghton FD. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation. 2006;74(1):11-8.
Leese HJ, Barton AM. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil. 1984;72(1):9-13.
Pantaleon M, Kaye PL. Glucose transporters in preimplantation development. Rev Reprod. 1998;3(2):77-81.
Van Blerkom J. Mitochondria in early mammalian development. Semin Cell Dev Biol. 2009;20(3):354-64.
Lane M, Gardner DK. Differential regulation of mouse embryo development and viability by amino acids. J Reprod Fertil. 1997;109(1):153-64.
Gopichandran N, Leese HJ. Metabolic characterization of the bovine blastocyst, inner cell mass, trophectoderm and blastocoel fluid. Reproduction. 2003;126(3):299-308.
Houghton FD, Hawkhead JA, Humpherson PG, et al. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum Reprod. 2002;17(4):999-1005.
Gonzalez IM, Martin PM, Burdsal C, et al. Leucine and arginine regulate trophoblast motility through mTOR-dependent and independent pathways in the preimplantation mouse embryo. Dev Biol. 2012;361(2):286-300.
Ikeda S, Sugimoto M, Kume S. Importance of methionine metabolism in morula-to-blastocyst transition in bovine preimplantation embryos. J Reprod Dev. 2012;58(1):91-7.
Tang S, Fang Y, Huang G, et al. Methionine metabolism is essential for SIRT1-regulated mouse embryonic stem cell maintenance and embryonic development. EMBO J. 2017;36(21):3175-93.
Yamada M, Takanashi K, Hamatani T, et al. A medium-chain fatty acid as an alternative energy source in mouse preimplantation development. Sci Rep. 2012;2:930.
Lee JH, Laronde S, Collins TJ, et al. Lineage-specific differentiation is influenced by state of human pluripotency. Cell Rep. 2017;19(1):20-35.
Sone M, Morone N, Nakamura T, et al. Hybrid cellular metabolism coordinated by zic3 and esrrb synergistically enhances induction of naive pluripotency. Cell Metab. 2017;25(5):1103-17.
Carey BW, Finley LW, Cross JR, et al. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518(7539):413-6.
Folmes CD, Nelson TJ, Martinez-Fernandez A, et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14(2):264-71.
Moussaieff A, Rouleau M, Kitsberg D, et al. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015;21(3):392-402.
Ware CB, Wang L, Mecham BH, et al. Histone deacetylase inhibition elicits an evolutionarily conserved self-renewal program in embryonic stem cells. Cell Stem Cell. 2009;4(4):359-69.
Melcer S, Hezroni H, Rand E, et al. Histone modifications and lamin A regulate chromatin protein dynamics in early embryonic stem cell differentiation. Nat Commun. 2012;3:910.
Hezroni H, Tzchori I, Davidi A, et al. H3K9 histone acetylation predicts pluripotency and reprogramming capacity of ES cells. Nucleus. 2011;2(4):300-9.
Gu W, Gaeta X, Sahakyan A, et al. Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell. 2016;19(4):476-90.
Shyh-Chang N, Locasale JW, Lyssiotis CA, et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339(6116):222-6.
Wang L, Zhang T, Wang L, et al. Fatty acid synthesis is critical for stem cell pluripotency via promoting mitochondrial fission. Embo J. 2017;36(10):1330-47.
Cliff TS, Wu T, Boward BR, et al. MYC controls human pluripotent stem cell fate decisions through regulation of metabolic flux. Cell Stem Cell. 2017;21(4):502-16.
Lu V, Dahan P, Ahsan FM, et al. Mitochondrial metabolism and glutamine are essential for mesoderm differentiation of human pluripotent stem cells. Cell Res. 2019;29(7):596-8.
Song C, Xu F, Ren Z, et al. Elevated exogenous pyruvate potentiates mesodermal differentiation through metabolic modulation and AMPK/mTOR pathway in human embryonic stem cells. Stem Cell Reports. 2019;13(2):338-51.
Xu XQ, Graichen R, Soo SY, et al. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation. 2008;76(9):958-70.
Freund C, Ward-van Oostwaard D, Monshouwer-Kloots J, et al. Insulin redirects differentiation from cardiogenic mesoderm and endoderm to neuroectoderm in differentiating human embryonic stem cells. Stem Cells. 2008;26(3):724-33.
Lee J, Kim MS. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res Clin Pract. 2007;77(1):S49-57.
Estaras C, Hsu HT, Huang L, et al. YAP repression of the WNT3 gene controls hESC differentiation along the cardiac mesoderm lineage. Genes Dev. 2017;31(22):2250-63.
Beyer TA, Weiss A, Khomchuk Y, et al. Switch enhancers interpret TGF-beta and Hippo signaling to control cell fate in human embryonic stem cells. Cell Rep. 2013;5(6):1611-24.
Cox AG, Hwang KL, Brown KK, et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol. 2016;18(8):886-96.
Cox A, Tsomides A, Yimlamai D, et al. Yap regulates glucose utilization and sustains nucleotide synthesis to enable organ growth. EMBO J. 2018;37(22):e100294.
Jellusova J, Cato MH, Apgar JR, et al. Gsk3 is a metabolic checkpoint regulator in B cells. Nat Immunol. 2017;18(3):303-12.
Theeuwes WF, Gosker HR, Langen RCJ, et al. Inactivation of glycogen synthase kinase-3beta (GSK-3beta) enhances skeletal muscle oxidative metabolism. Biochim Biophys Acta. 2017;1863(12):3075-86.
Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56(2):130-40.
Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial atp production immediately after birth. Am J Physiol. 1991;261(6):H1698-705.
Guimaraes-Camboa N, Stowe J, Aneas I, et al. HIF1alpha represses cell stress pathways to allow proliferation of hypoxic fetal cardiomyocytes. Dev Cell. 2015;33(5):507-21.
Neary MT, Ng KE, Ludtmann MH, et al. Hypoxia signaling controls postnatal changes in cardiac mitochondrial morphology and function. J Mol Cell Cardiol. 2014;74:340-52.
Larsson NG, Wang JM, Wilhelmsson H, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nature Genetics. 1998;18(3):231-6.
Hom JR, Quintanilla RA, Hoffman DL, et al. The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev Cell. 2011;21(3):469-78.
Chung S, Dzeja PP, Faustino RS, et al. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4 Suppl 1:S60-7.
Cho SW, Park JS, Heo HJ, et al. Dual modulation of the mitochondrial permeability transition pore and redox signaling synergistically promotes cardiomyocyte differentiation from pluripotent stem cells. J Am Heart Assoc. 2014;3(2):e000693.
Puente BN, Kimura W, Muralidhar SA, et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157(3):565-79.
Denning C, Borgdorff V, Crutchley J, et al. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim Biophys Acta. 2016;1863(7):1728-48.
Eder A, Vollert I, Hansen A, et al. Human engineered heart tissue as a model system for drug testing. Adv Drug Deliv Rev. 2016;96:214-24.
Rupert CE, Irofuala C, Coulombe KLK. Practical adoption of state-of-the-art hiPSC-cardiomyocyte differentiation techniques. PLoS One. 2020;15(3):e0230001.
Lin B, Lin X, Stachel M, et al. Culture in glucose-depleted medium supplemented with fatty acid and 3,3',5-triiodo-l-thyronine facilitates purification and maturation of human pluripotent stem cell-derived cardiomyocytes. Front Endocrinol (Lausanne). 2017;8:253.
Nakano H, Minami I, Braas D, et al. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. Elife. 2017;6:e29330.
Yang P, Chen X, Kaushal S, et al. High glucose suppresses embryonic stem cell differentiation into cardiomyocytes: high glucose inhibits ES cell cardiogenesis. Stem Cell Res Ther. 2016;7(1):187.
Mills RJ, Titmarsh DM, Koenig X, et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci USA. 2017;114(40):E8372-81.
Birket MJ, Casini S, Kosmidis G, et al. PGC-1alpha and reactive oxygen species regulate human embryonic stem cell-derived cardiomyocyte function. Stem Cell Reports. 2013;1(6):560-74.
Hu D, Linders A, Yamak A, et al. Metabolic maturation of human pluripotent stem cell-derived cardiomyocytes by inhibition of HIF1alpha and LDHA. Circ Res. 2018;123(9):1066-79.
Yang X, Rodriguez ML, Leonard A, et al. Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. 2019;13(4):657-68.
Chanthra N, Abe T, Miyamoto M, et al. A novel fluorescent reporter system identifies laminin-511/521 as potent regulators of cardiomyocyte maturation. Sci Rep. 2020;10(1):4249.