Corresponding author: Shuichi Ito, itoshu@yokohama-cu.ac.jp
DOI: 10.31662/jmaj.2020-0115
Received: December 16, 2020
Accepted: June 2, 2021
Advance Publication: September 13, 2021
Published: October 15, 2021
Cite this article as:
Nozawa H, Ogura M, Miyasaka M, Suzuki H, Ishikura K, Ishiguro A, Ito S. Ultrasonography as a Diagnostic Support Tool for Childhood Takayasu Arteritis Referred to as Fever of Unknown Origin: Case Series and Literature Review. JMA J. 2021;4(4):358-366.
Introduction: Childhood Takayasu arteritis (c-TA) often shows nonspecific symptoms, such as fever of unknown origin (FUO). Delay of diagnosis may result in organ dysfunction by arterial occlusion; therefore, early diagnosis is very important. Although ultrasonography is the first-line screening tool for children with FUO, its diagnostic efficacy of evaluation of systemic arteries in c-TA that presents as FUO remains unclear. We evaluated the suitability of ultrasonography evaluation that included systemic vessels for the early diagnosis of c-TA initially presenting as FUO.
Methods: We review five patients who received a diagnosis of c-TA in our institution and also performed a literature review regarding TA cases with FUO and diagnosed on the basis of initial ultrasonography.
Results: As in our cases, the median period from onset to diagnosis was 25 days (interquartile range [IQR], 21-35). Comparing the initial ultrasonography findings with later contrast-enhanced computed tomography (CECT) findings in the abdominal aorta, celiac artery, superior mesenteric artery, bilateral renal arteries, and bilateral common carotid arteries, the concordance rate between ultrasonography and CECT was moderate (Kappa coefficient was 0.50). All the patients were successfully treated without severe vascular damage. The literature review revealed 12 articles; although 9 of the 13 patients did not show the characteristic features (such as blood pressure discrepancy, bruit, or pulse deficiency), the median time to diagnosis was still 5 months (IQR, 3-12).
Conclusions: During initial screening for patients with FUO, ultrasonography including evaluation of systemic vessels could contribute to earlier diagnosis of c-TA.
Key words: Takayasu arteritis, pediatrics, diagnostic imaging, fever of unknown origin, ultrasonography
Takayasu arteritis (TA) is a large vessel vasculitis affecting the aorta and its major branches. Childhood TA (c-TA) is a very rare disease, with an annual incidence in the pediatric population of approximately 2/1,000,000 (1). Additionally, during the early stages, its typical clinical manifestations (pulseless upper extremities, leg claudication, and fainting) are often lacking. Furthermore, c-TA often presents as a fever of unknown origin (FUO). Kasai et al. reported that 4.8% of children with FUO eventually received a diagnosis of c-TA (2). Additionally, approximately 80% of patients with c-TA actually demonstrated fever (3), although that is a relatively minor symptom in adult TA (<34.7%) (4). Early diagnosis of c-TA is challenging for pediatricians but is very important because delay in diagnosis and intervention may result in vascular stenosis, dilation, and obstruction, which are often followed by organ dysfunction.
The appropriate criteria from the American College of Radiology state that ultrasonography may be appropriate for the imaging modality evaluating pediatric patients with FUO (5) because it is free from radiation exposure and the need for sedation. The recommendations from the European League Against Rheumatism (EULAR) mention the early diagnosis by ultrasonography (6). However, the systematic review of the diagnosis of large vessel vasculitis states that there was no high-quality literature evaluating the usefulness of ultrasonography in the diagnosis of TA (7). If we could detect abnormal imaging findings of TA among patients with FUO with ultrasonography screening, we would immediately select essential radiological diagnostic procedures for definitive diagnosis of TA, such as contrast-enhanced computed tomography (CECT). To the best of our knowledge, no literature has reviewed the contribution of ultrasonography screening to the diagnosis of c-TA in patients with FUO. On the basis of the experience of pediatric cases diagnosed as c-TA in patients with FUO, we evaluated using case literature review the suitability of ultrasonography evaluation that included systemic vessels for the early diagnosis of c-TA initially presenting as FUO.
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the National Center for Child Health and Development on July 27, 2017 (number 1493), and written informed consent was obtained from all patients.
We included all patients who were referred as FUO to the National Center for Child Health and Development in Tokyo between March 2002 and March 2016 and who finally received a diagnosis of c-TA. Definitive diagnosis was based on EULAR, Paediatric Rheumatology International Trials Organisation (PRINTO), and Paediatric Rheumatology European Society (PRES) criteria for TA (8). We excluded patients who already had suspected c-TA by several radiological procedures performed in previous hospitals and were referred for treatment of c-TA. We investigated their clinical findings, including sex, age of onset, laboratory examinations (C-reactive protein level and erythrocyte sedimentation rate), duration from onset to diagnosis, Indian Takayasu Activity Score 2010 (ITAS2010) at diagnosis and at the latest ultrasonography follow-up date until July 2020, treatment, and Vasculitis Damage Index (VDI) at the latest ultrasonography follow-up date until July 2020.
We also compared the ultrasonography and CECT findings of our patients regarding the following seven vessels: abdominal aorta (AAo), celiac artery, superior mesenteric artery (SMA), right and left renal arteries, and right and left common carotid arteries (CCAs).
We searched PubMed, Ovid/MEDLINE, Embase, and Cochrane library databases for previously published studies until August 31, 2020. In each database, the search query was the following: (“Takayasu arteritis”[MeSH Terms] OR “takayasu*”[Title/Abstract]) AND (“ultrasonography”[MeSH Terms] OR (“ultrasound”[Title/Abstract] OR “ultrasonography”[Title/Abstract] OR “sonography”[Title/Abstract])) in PubMed and Ovid/MEDLINE and (‘aortic arch syndrome’/exp OR ‘takayasu*’:ab,ti) AND (‘echography’/exp OR ‘ultrasound’:ab,ti OR ‘ultrasonography’:ab,ti OR ‘sonography’:ab,ti) AND [english]/lim AND [embase]/lim in Embase, ([Takayasu Arteritis] explode all trees) AND ([Ultrasonography] explode all trees) in Cochrane library. English language filter was activated in PubMed and Ovid/MEDLINE. We also performed a manual search of reference literature from the full text available studies.
Case reports about the diagnosis of TA by the initial ultrasonography for patients with FUO were included. Review articles and the literature lacking the correct clinical course for individual patients were excluded.
During this study period, five patients received a diagnosis of c-TA. Table 1 shows the summary of the five patients. All patients had suspected TA by the initial screening ultrasonography and then received a definitive diagnosis on the follow-up CECT. They fulfilled the EULAR-PRINTO-PRES diagnostic criteria. The median age at onset was 11 years (interquartile range [IQR], 9-12 years). The median duration from onset to diagnosis was 25 days (IQR, 21-35 days). The median value of ITAS2010 at diagnosis was 3 (IQR, 1-9). All patients received immunosuppressive treatment, and the value of ITAS2010 decreased to 0 at the most recent ultrasonography follow-up date. The highest value of VDI among five patients was 2.
Table 1. Summary of Clinical Manifestations of Five Patients.
| Case No. | Sex/Age at onset | Clinical symptoms other than fever | Applicable number to EULAR-PRINTO-PRES criteria/presence of bruit | Laboratory data at diagnosis | Duration from onset to diagnosis (days) | ITAS2010 at diagnosis | Treatment | Follow-up status at most recent US date | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP (mg/dL) | ESR (mm/h) | Induction therapy | Present therapy | ITAS2010 | VDI | Follow-up period (months) | ||||||
| 1 | F/12 years | Knee pain | 3/Yes | 7.8 | 129 | 25 | 9 | MPT | PSL/AZP/MTX | 0 | 1 | 53 |
| 2 | M/9 years | Abdominal pain | 1/No | 8.2 | 132 | 21 | 1 | MPT | PSL/AZP | 0 | 1 | 55 |
| 3 | M/12 years | Abdominal pain | 2/Yes | 10.4 | 106 | 15 | 3 | MPT/IVCY | MMF | 0 | 2 | 70 |
| 4 | F/11 years | Lymphadenopathy | 4/Yes | 3.0 | 113 | 35 | 11 | MPT/IVCY | None | 0 | 1 | 91 |
| 5 | F/5 months | Cough | 2/No | 11.5 | 132 | 37 | 0 | MPT/IVCY | None | 0 | 0 | 60 |
| AZP, azathioprine; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; F, female; IVCY, intravenous cyclophosphamide therapy; ITAS, Indian Takayasu activity score, M, male; MMF, mycophenolate mofetil; MPT, methylprednisolone pulse therapy; MTX, methotrexate; PRES, Pediatric Rheumatology European Society; PRINTO, Pediatric Rheumatology International Trials Organization; PSL, prednisolone; VDI, vasculitis damage index | ||||||||||||
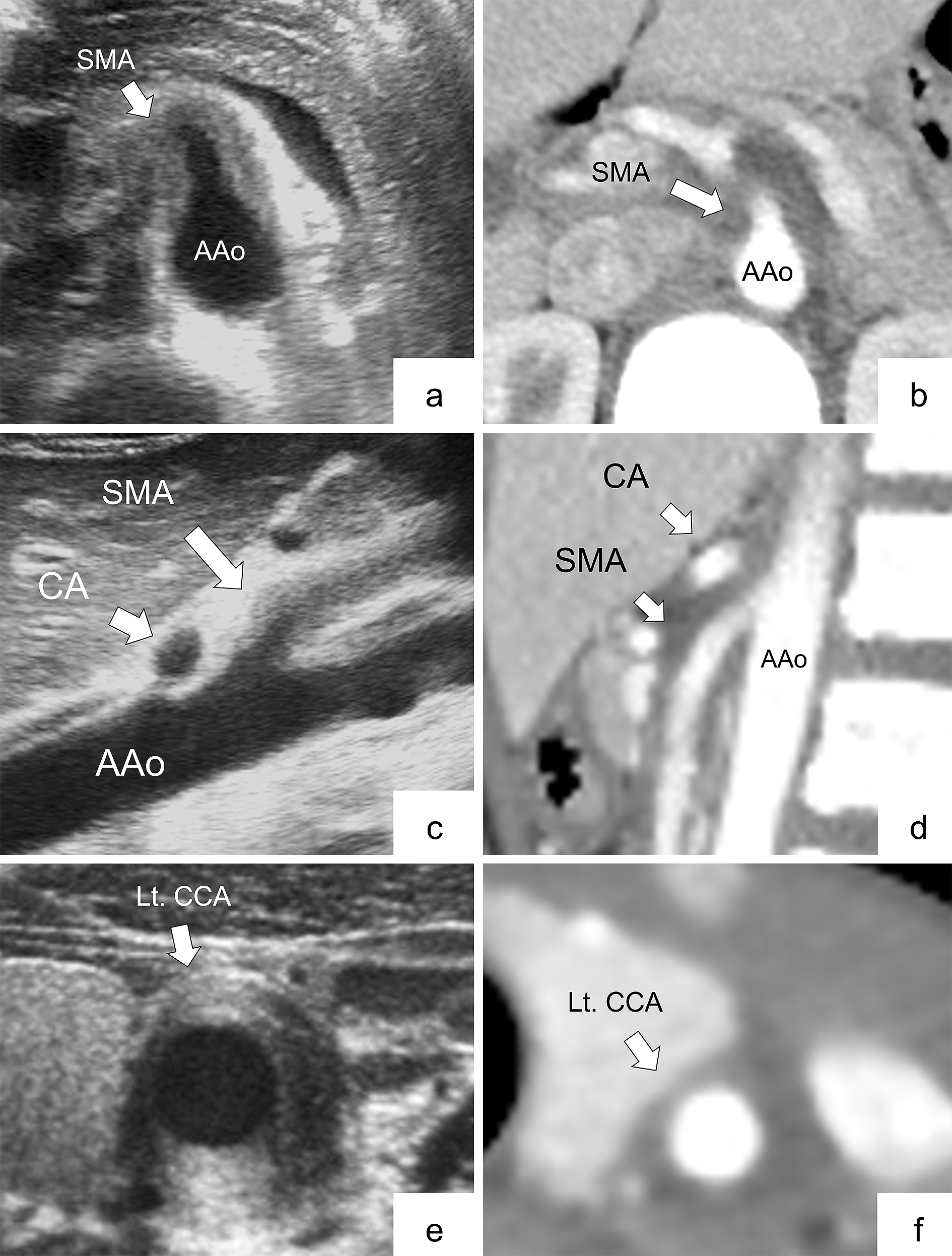
Table 2 shows the radiological findings of ultrasonography and CECT for seven arteries. Figure 1 (panels a, b, c, d, e, and f) shows the representative ultrasonograms. All findings were classified as follows: 9 arteries were positive on both ultrasonography and CECT (6 abdominal and 3 cervical), 2 were positive on ultrasonography alone (1 abdominal and 1 cervical), 6 were positive on CECT alone (4 abdominal and 2 cervical), and 16 were negative on both ultrasonography and CECT (14 abdominal and 2 cervical). For patient 5, bilateral CCAs were not evaluated by either ultrasonography or CECT. There was moderate agreement between ultrasonography and CECT assessment on all arteries and abdominal arteries (Kappa coefficients were 0.50 and 0.56).
Table 2. Arterial Findings Compared between Ultrasonography and Contrast-enhanced Computed Tomography.
| Case No. | Abdominal arteries (US/CECT) | Cervical arteries (US/CECT) | US undetectable lesions detected by CECT | |||||
|---|---|---|---|---|---|---|---|---|
| AAo | CA | Right RA | Left RA | SMA | Right CCA | Left CCA | ||
| 1 | −/+ | −/− | −/+ | −/− | +/+ | −/− | +/+ | Wall thickening of the left subclavian artery and trunk of right renal artery |
| 2 | −/- | −/− | −/− | −/− | −/− | −/+ | +/+ | None |
| 3 | −/− | +/+ | −/− | −/− | +/+ | −/− | +/+ | None |
| 4 | −/+ | −/+ | −/− | −/− | +/+ | +/− | −/+ | None |
| 5 | +/+ | +/+ | −/− | −/− | +/− | NA | NA | None |
| AAo, abdominal aorta; CA, celiac artery; CCA, common carotid artery; CECT, contrast-enhanced computed tomography; NA, not available; RA, renal artery; SMA, superior mesenteric artery; US, ultrasonography | ||||||||
According to the search strategy, inclusion and exclusion criteria, 12 articles including 13 cases were reviewed (9), (10), (11), (12), (13), (14), (15), (16), (17), (18), (19), (20). Figure 2 shows a flowchart depicting the article screening and selection. Table 3 shows the summary of all patients in the reviewed articles. Only three of 13 patients showed specific manifestations, including bruit, pulse deficit, or blood pressure discrepancy. The median duration from onset to diagnosis was 5 months (IQR, 3-12 months).
Table 3. Summary of Literature Review on TA Cases Detected by Initial US Screening among Patients with Fever of Unknown Origin.
| Study | Sex/Age at onset (years) | Clinical symptoms other than fever | Applicable number to EULAR-PRINTO-PRES criteria/Presence of a bruit | Laboratory data at diagnosis | Duration from onset to diagnosis (months) | US/Definitive image | Modality for definitive diagnosis | Follow-up period (months) | Present therapy | Relapse | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP (mg/dL) | ESR (mm/h) | Abdominal | Cervical/Thoracic | |||||||||
| Tachibana et al | F/20 | Pain throughout entire body | 1/No | 7.5 | 99 | 4 | NA/+ | +/+ | CTA | 6 | PSL/MTX | Yes |
| Goel et al | M/18 | Dyspnea | 1/No | 6.0 | 35 | 6 | +/+ | +/+ | CTA | 3 | PSL/MTX | No |
| Sasae et al | F/20 | Periumbilical pain | 2/No | 9.1 | 119 | 3 | +/+ | +/+ | CTA | 4 | PSL | No |
| Sada et al | F/19 | Malaise | 1/No | 31.2 | 145 | 6 | NA/NA | +/+ | CTA | 24 | PSL/MTX | No |
| Agostinis et al | M/75 | Cough and neck pain | 1/No | 15.1 | 85 | 3 | NA/+ | +/+ | FDG-PET | 6 | PSL/MTX | No |
| Gupta et al | F/20 | Painful nodular skin lesions | 2/No | 15.2 | 77 | 12 | +/+ | NA/+ | MRA | NA | PSL | No |
| Ahasan et al | F/20 | Pain in all four limbs | 2/No | 1.2 | 62 | 2 | +/NA | NA/NA | NA | NA | PSL | NA |
| Thai et al | F/14 | Pain in left upper extremity | 4/Yes | 21000.0 | 154 | 4 | NA/NA | +/NA | MRA | NA | PSL | NA |
| Skoura et al | F/54 | None | 1/No | 2.0 | 80 | NA | +/NA | NA/+ | FDG-PET | 6 | PSL | NA |
| Uthman et al | F/28 | None | 1/No | NA | 150 | 18 | NA/NA | +/+ | DSA | 5 | PSL/MTX | NA |
| KiŞla Ekİncİ et al | F/12 | Painful lesion on the face | 1/No | 14.8 | 116 | 2 | +/+ | +/+ | MRA | 18 | PSL/MTX/MM F | Yes |
| Schmidt et al | F/38 | Joint pain | 1/No | 3.6 | 92 | 12 | NA/NA | +/+ | DSA | 14 | PSL/AZP | No |
| F/27 | Joint pain | 1/No | 24.6 | 120 | 36 | NA/NA | +/+ | DSA | 8 | PSL/MTX | Yes | |
| AZP, azathioprine; CTA, computed tomography angiography; CRP, C-reactive protein; DSA, digital subtraction angiography; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; F, female; FDG-PET, [18F]-fluorodeoxyglucose positron emission tomography; M, male; MMF, mycophenolate mofetil; MRA, magnetic resonance angiography; MTX, methotrexate; NA, not available; PRES, Pediatric Rheumatology European Society; PRINTO, Pediatric Rheumatology International Trials Organization; PSL, prednisolone; US, ultrasonography | ||||||||||||
According to the present study, it is important for early diagnosis of c-TA to perform ultrasonography screening that includes large systemic vessels (as many as we can observe) among children with FUO. Detection of ultrasonography abnormalities may lead to confirmative examinations, such as via CECT, and early diagnosis of c-TA contributed to favorable patient outcomes. The diagnosis of TA is generally made by a combination of imaging modalities. Our study showed that ultrasonography was able to detect abnormal arteries in the abdomen and neck regions with a moderate concordance rate compared with CECT. In the early stage of TA, patients show only nonspecific symptoms, such as persistent fever. Additionally, in the so-called “pre-pulseless phase” of vascular stenosis, pulse deficit or blood pressure discrepancy, which are hallmarks of TA, may be lacking (21). However, both in our patients and in cases from the review articles, the median duration from onset to definitive diagnosis tends to be shorter than those stated in the previous studies (22), (23), (24), (25). Furthermore, ultrasonography is minimally invasive (free from radiation exposure or sedation) and low in cost and provides satisfactory access to images. Thus, it is preferable as a screening and diagnostic support tool for c-TA among children with FUO.
There are two reasons why ultrasonography evaluation of systemic arteries, particularly including those in the abdomen, is suitable for c-TA patients among children with FUO: first, different distribution of arterial involvement in c-TA compared with that in adult TA and, second, favorable body habitus of children for ultrasonography screening.
Recent reports revealed that c-TA is more likely to demonstrate subphrenic aorta involvement rather than in intrathoracic or suprathoracic regions (26), (27), (28). Our results were also consistent with this presentation. A retrospective cohort study of c-TA in the United States reported that the frequency of abdominal involvement was 52.4% in the AAo and 42.9% in the SMA, and (in the thorax and neck) 38.1% in the right CCA and 42.9% in the left CCA (29). Feng et al. reported arterial involvement in 11 patients with c-TA. All 11 patients had involvement of the AAo and its branches, but only three (27.2%) had involvement of the CCAs (30).
As mentioned above, the body habitus of children is favorable for abdominal ultrasonography screening compared with that of adults. They have a thinner abdominal wall and less fat compared with adults (31), thus enabling higher-resolution ultrasonography images. The “macaroni sign,” which is a long, smooth, homogeneous, and moderately echogenic circumferential thickening of the cervical arteries on the transverse section, specific for early TA (32), (33), (34), is similarly observed in the abdominal region in our patients. In adults, abdominal arteries are often obscured because ultrasonography cannot visualize them because of a larger physique with thick subcutaneous tissue or large volumes of bowel gas. Thus, in adult TA, the efficacy of ultrasonography is mainly limited to CCAs (35), (36), (37). Among the cases in the review articles, the patients’ median age whose involvement of the abdominal arteries could be detected by ultrasonography was 20 years (range, 12-54 years), which tended to be young.
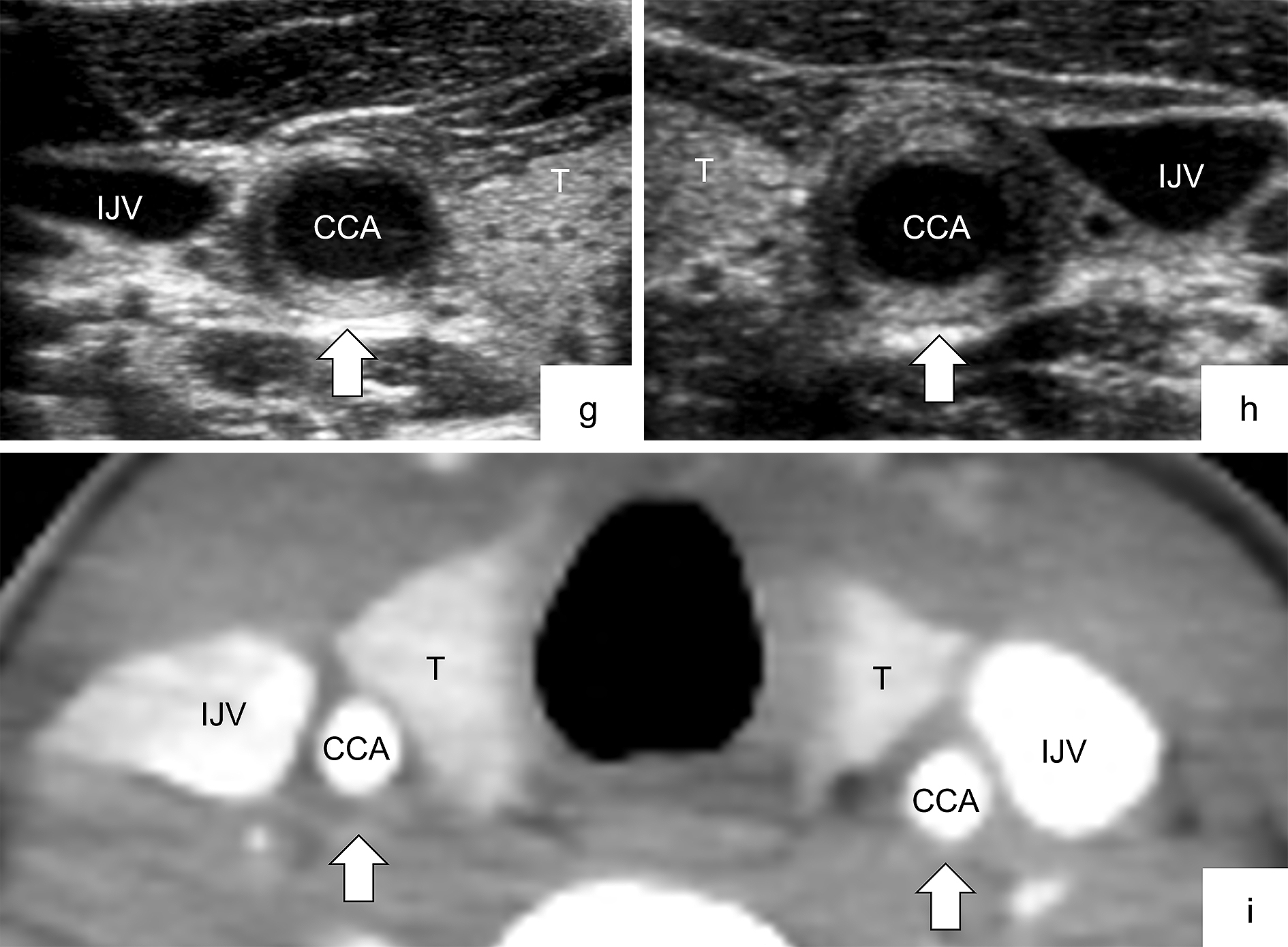
Ultrasonography findings did not completely correspond with CECT findings in the cervical regions. The Kappa coefficient, which indicates the concordance rate between ultrasonography and CECT, also tended to be slightly higher in the abdominal region only than in the abdominal and cervical regions together. It may suggest that cervical CECT may be inferior to abdominal CECT in diagnostic accuracy. As subcutaneous fat and tissue during childhood are significantly thinner in the neck area than in adults, it is difficult to distinguish the arterial wall from the periarterial soft tissue in CECT. Additionally, technical problems, such as motion artifact or inaccurate scan setting (timing and slice thickness), can affect image quality. These also apply to magnetic resonance imaging (MRI) and may be a common caveat particularly in children who are small and difficult to immobilize for a long time. Furthermore, if the arterial abnormality (i.e., wall thickening) is localized in a small section of the carotid artery, as shown in Figure 3 (panels g, h, and i), CECT may not differentiate such small vascular abnormalities. As CECT or MRI is not always a definitive imaging modality, additional ultrasonography examination should be considered when c-TA is suspected.
Although the results of our study encourage initial ultrasonography screening of systemic arteries to find c-TA in children with FUO, our study has several limitations. First, this study was a retrospective analysis with small numbers of patients at a single center. Second, TA is a more well-known and prevalent disease among Japanese and other Asian populations than among those of Europe and the United States. Third, ultrasonography is dependent on observers’ skills and cannot visualize the proximal subclavian artery and thoracic aorta because of the interference of the costal bones and the normal presence of air in the lung. Finally, we compared only ultrasonography and CECT but did not include contrast-enhanced ultrasonography (CEUS) or MRI. Recently, CEUS and MRI have been added as effective tools for the diagnosis and assessment of disease activity in TA (32), (37), (38), (39). CEUS has not yet been introduced in our institution. When screening for FUO, we performed CECT as a secondary survey prior to MRI to search for fever sources other than arteritis. Considering that some patients at the susceptible age for TA do not always require sedation during short-term MRI, in the future, we intend to perform MRI focusing on abnormal arteries to confirm the diagnosis of TA.
Ultrasonography is preferable as a screening and diagnostic support tool for c-TA referred to as pediatric patients with FUO. For early diagnosis of c-TA, it is important to perform ultrasonography screening that includes large systemic vessels (as many as we can observe) among children with FUO.
Shuichi Ito has the following conflicts of interest relevant to this article to disclose: an advisory fee from Takeda Pharmaceutical, and the lecture fees from Chugai Pharmaceutical, Mitsubishi Tanabe Pharma, Pfizer, and Takeda Pharmaceutical, and research funding from Chugai Pharmaceutical and Mitsubishi Tanabe Pharma.
Kenji Ishikura has the following conflicts of interest relevant to this article to disclose: honoraria from Asahi Kasei Pharma, Chugai Pharmaceutical, Novartis Pharma, and Zenyaku Kogyo, and research funding from Chugai Pharmaceutical and Zenyaku Kogyo.
We thank the medical editor from the Division of Education for Clinical Research, National Center for Child Health and Development for editing this article.
All authors contributed to the conception and design of the study. Hisataka Nozawa provided material preparation, data collection, interpretation, and participated in the design, and revision of the review and wrote the first draft of the manuscript. Mikiko Miyasaka revised figures and figure legends. Hiromichi Suzuki developed the search strategy for searches. Shuichi Ito supervised the entire process. All authors commented on previous versions of the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work.
Shuichi Ito is one of the Editors of JMA Journal and on the journal’s Editorial Staff. He was not involved in the editorial evaluation or decision to accept this article for publication at all.
This study was performed in line with the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in this study and their parents. The participants and their parents consented to the submission of the case series to the journal. Approval was granted by the Ethics Committee of the National Center for Child Health and Development on July 27, 2017 (number 1493).
Russo RAG, Katsicas MM. Takayasu arteritis. Front Pediatr. 2018;6:265.
Kasai K, Mori M, Hara R, et al. National survey of childhood febrile illness cases with fever of unknown origin in Japan. Pediatr Int. 2011;53(4):421-5.
Maeda M, Kobayashi M, Okamoto S, et al. Aortitis syndrome in children: clinical observation of 35 cases in Japan. Acta Paediatr Jpn. 1997;39(2):280-4.
Watanabe Y, Miyata T, Tanemoto K. Current clinical features of new patients with Takayasu arteritis observed from cross-country research in Japan: age and sex specificity. Circulation. 2015;132(18):1701-9.
Westra SJ, Karmazyn BK, Alazraki AL, et al. ACR appropriateness criteria fever without source or unknown origin―child. J Am Coll Radiol. 2016;13(8):922-30.
Bardi M, Diamantopoulos AP. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice summary. Radiol Med. 2019;124(10):965-72.
Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612.
Ozen S, Pistorio A, Iusan SM, et al. EULAR/PRINTO/PRES criteria for Henoch-Schonlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69(5):798-806.
Tachibana M, Mukouhara N, Hirami R, et al. Ultrasonography is convenient and useful for assessment and follow-up of Takayasu’s arteritis. J Med Ultrason (2001). 2014;41(3):365-9.
Goel PK, Moorthy N, Kumar S. The role of noninvasive imaging in early diagnosis of clinically masked prepulseless inflammatory phase of Takayasu’s arteritis. Echocardiography. 2012;29(1):59-63.
Sasae Y, Morita Y, Sakuta T, et al. Abdominal pain as the initial presentation of Takayasu arteritis. Mod Rheumatol. 2008;18(5):496-8.
Sada E, Kohno M, Iwamasa K, et al. Clinical usefulness of multiplanar reconstruction images obtained by multi-slice computed tomographic angiography for early-stage Takayasu’s arteritis. Mod Rheumatol. 2004;14(3):245-9.
Agostinis P, Antonello RM, Orsaria M, et al. Isoniazid-induced Takayasu arteritis remission. Infez Med. 2019;27(4):436-40.
Gupta M, Singh K, Lehl SS, et al. Recurrent erythema nodosum: a red flag sign of hidden systemic vasculitis. BMJ Case Rep. 2013;2013:bcr2013009507.
Ahasan HAMN, Alam B, Chowdhury MH, et al. Takayasu’s arteritis in association with tuberculosis in a young woman. Pak J Med Sci. 2009;25(6):1009-11.
Tsai MJ, Lin SC, Wang JK, et al. A patient with familial Takayasu’s arteritis presenting with fever of unknown origin. J Formos Med Assoc. 1998;97(5):351-3.
Skoura E, Giannopoulou C, Keramida G, et al. A case of fever of unknown origin: (18)F-FDG-PET/CT findings in Takayasu’s arteritis. Hell J Nucl Med. 2008;11(3):172-4.
Uthman IW, Bizri AR, Hajj Ali RA, et al. Takayasu’s arteritis presenting as fever of unknown origin: report of two cases and literature review. Semin Arthritis Rheum. 1999;28(4):280-5.
KiŞla Ekİncİ RM, Balci S, PİŞkİn FC, et al. Pre-pulseless Takayasu arteritis in a child represented with prolonged fever of unknown origin and successful management with concomitant mycophenolate mofetil and infliximab. Arch Rheumatol. 2020;35(2):278-82.
Schmidt W, Nerenheim A, Seipelt E, et al. Diagnosis of early Takayasu arteritis with sonography. Rheumatology (Oxford). 2002;41(5):496-502.
Yotsuyanagi H, Chikatsu N, Kaneko Y, et al. Takayasu’s arteritis in prepulseless stage manifesting lymph node swelling and hepatosplenomegaly. Intern Med. 1995;34(5):455-9.
Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919-29.
Sahin S, Hopurcuoglu D, Bektas S, et al. Childhood-onset Takayasu arteritis: a 15-year experience from a tertiary referral center. Int J Rheum Dis. 2019;22(1):132-9.
Aeschlimann FA, Barra L, Alsolaimani R, et al. Presentation and disease course of childhood-onset versus adult-onset Takayasu arteritis. Arthritis Rheumatol. 2019;71(2):315-23.
Clemente G, Silva CA, Sacchetti SB, et al. Takayasu arteritis in childhood: misdiagnoses at disease onset and associated diseases. Rheumatol Int. 2018;38(6):1089-94.
Clemente G, Hilario MO, Len C, et al. Brazilian multicenter study of 71 patients with juvenile-onset Takayasu’s arteritis: clinical and angiographic features. Rev Bras Reumatol Engl Ed. 2016;56(2):145-51.
Mathew AJ, Goel R, Kumar S, et al. Childhood-onset Takayasu arteritis: an update. Int J Rheum Dis. 2016;19(2):116-26.
Goel R, Kumar TS, Danda D, et al. Childhood-onset Takayasu arteritis―experience from a tertiary care center in South India. J Rheumatol. 2014;41(6):1183-9.
Szugye HS, Zeft AS, Spalding SJ. Takayasu arteritis in the pediatric population: a contemporary United States-based single center cohort. Pediatr Rheumatol Online J. 2014;12:21.
Feng Y, Tang X, Liu M, et al. Clinical study of children with Takayasu arteritis: a retrospective study from a single center in China. Pediatr Rheumatol Online J. 2017;15(1):29.
Siegel MJ. Pediatric sonography. 4th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2011. 725 p.
Schmidt WA. Role of ultrasound in the understanding and management of vasculitis. Ther Adv Musculoskelet Dis. 2014;6(2):39-47.
Chaubal N, Dighe M, Shah M. Sonographic and color Doppler findings in aortoarteritis (Takayasu arteritis). J Ultrasound Med. 2004;23(7):937-44.
Matsunaga N, Hayashi K, Sakamoto I, et al. Takayasu arteritis: protean radiologic manifestations and diagnosis. Radiographics. 1997;17(3):579-94.
Kissin EY, Merkel PA. Diagnostic imaging in Takayasu arteritis. Curr Opin Rheumatol. 2004;16(1):31-7.
Pipitone N, Versari A, Salvarani C. Role of imaging studies in the diagnosis and follow-up of large-vessel vasculitis: an update. Rheumatology (Oxford). 2008;47(4):403-8.
Gotway MB, Araoz PA, Macedo TA, et al. Imaging findings in Takayasu’s arteritis. AJR Am J Roentgenol. 2005;184(6):1945-50.
Czihal M, Lottspeich C, Hoffmann U. Ultrasound imaging in the diagnosis of large vessel vasculitis. Vasa. 2017;46(4):241-53.
Soliman M, Laxer R, Manson D, et al. Imaging of systemic vasculitis in childhood. Pediatr Radiol. 2015;45(8):1110-25.